GadodiamideCAS# 122795-43-1 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 122795-43-1 | SDF | Download SDF |
| PubChem ID | 60754 | Appearance | Powder |
| Formula | C16H28GdN5O9 | M.Wt | 591.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 16 mg/mL (27.04 mM; Need ultrasonic and warming) | ||
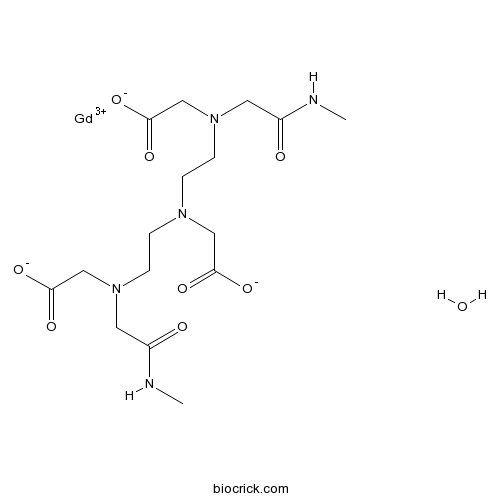
| Chemical Name | 2-[bis[2-[carboxylatomethyl-[2-(methylamino)-2-oxoethyl]amino]ethyl]amino]acetate;gadolinium(3+);hydrate | ||
| SMILES | CNC(=O)CN(CCN(CCN(CC(=O)NC)CC(=O)[O-])CC(=O)[O-])CC(=O)[O-].O.[Gd+3] | ||
| Standard InChIKey | XPCLDSMKWNNKOM-UHFFFAOYSA-K | ||
| Standard InChI | InChI=1S/C16H29N5O8.Gd.H2O/c1-17-12(22)7-20(10-15(26)27)5-3-19(9-14(24)25)4-6-21(11-16(28)29)8-13(23)18-2;;/h3-11H2,1-2H3,(H,17,22)(H,18,23)(H,24,25)(H,26,27)(H,28,29);;1H2/q;+3;/p-3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gadodiamide is a gadolinium-based contrast agent used in MR imaging procedures to assist in the visualization of blood vessels.
Target: Others
Gadodiamide(Omniscan) is a gadolinium-based contrast agent used in MR imaging procedures to assist in the visualization of blood vessels. Gadodiamide is a contrast medium for cranial and spinal magnetic resonance imaging (MRI) and for general MRI of the body after intravenous administration. The product provides contrast enhancement and facilitates visualisation of abnormal structures or lesions in various parts of the body including the central nervous system (CNS). A recent review takes the question of toxicity caused by loss of gadolinium from the complex. The challenge for nephrologists includes (a) evidence of transmetallation, such as gadolinium deposits in bone, increased urinary zinc excretion, iron-transferrin dissociation or 'spurious hypocalcemia' in exposed people [1]. References: | |||||

Gadodiamide Dilution Calculator

Gadodiamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6901 mL | 8.4507 mL | 16.9013 mL | 33.8026 mL | 42.2533 mL |
| 5 mM | 0.338 mL | 1.6901 mL | 3.3803 mL | 6.7605 mL | 8.4507 mL |
| 10 mM | 0.169 mL | 0.8451 mL | 1.6901 mL | 3.3803 mL | 4.2253 mL |
| 50 mM | 0.0338 mL | 0.169 mL | 0.338 mL | 0.6761 mL | 0.8451 mL |
| 100 mM | 0.0169 mL | 0.0845 mL | 0.169 mL | 0.338 mL | 0.4225 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gadodiamide is a gadolinium-based contrast agent used in MR imaging procedures to assist in the visualization of blood vessels.
- GSK2334470
Catalog No.:BCC4982
CAS No.:1227911-45-6
- ACTH (1-39)
Catalog No.:BCC6028
CAS No.:12279-41-3
- CEP-32496 hydrochloride
Catalog No.:BCC1468
CAS No.:1227678-26-3
- MNI-caged-NMDA
Catalog No.:BCC5888
CAS No.:1227675-52-6
- SCH 39166 hydrobromide
Catalog No.:BCC7317
CAS No.:1227675-51-5
- A 943931 dihydrochloride
Catalog No.:BCC7772
CAS No.:1227675-50-4
- StemRegenin 1 (SR1)
Catalog No.:BCC3637
CAS No.:1227633-49-9
- [D-Ala2]-Deltorphin II
Catalog No.:BCC5723
CAS No.:122752-16-3
- Deltorphin I
Catalog No.:BCC6233
CAS No.:122752-15-2
- Bi-linderone
Catalog No.:BCN6116
CAS No.:1227375-09-8
- Philanthotoxin 74
Catalog No.:BCC7478
CAS No.:1227301-51-0
- Liangshanin A
Catalog No.:BCN6115
CAS No.:122717-54-8
- Marinopyrrole A
Catalog No.:BCC4098
CAS No.:1227962-62-0
- Sabutoclax
Catalog No.:BCC2236
CAS No.:1228108-65-3
- 8-Geranyloxy-5,7-dimethoxycoumarin
Catalog No.:BCN6117
CAS No.:1228175-65-2
- MRS 2957 triethylammonium salt
Catalog No.:BCC6133
CAS No.:1228271-30-4
- H-D-Phe(4-F)-OH .HCl
Catalog No.:BCC3217
CAS No.:122839-52-5
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Cefoselis Sulfate
Catalog No.:BCC4769
CAS No.:122841-12-7
- Alosetron
Catalog No.:BCC1342
CAS No.:122852-42-0
- Alosetron (Z)-2-butenedioate
Catalog No.:BCC1343
CAS No.:122852-43-1
- Alosetron Hydrochloride
Catalog No.:BCC1344
CAS No.:122852-69-1
- Panaxyne
Catalog No.:BCN6462
CAS No.:122855-49-6
- GS-9620
Catalog No.:BCC1602
CAS No.:1228585-88-3
Incidence of Nephrogenic Systemic Fibrosis Using Gadobenate Dimeglumine in 1423 Patients With Renal Insufficiency Compared With Gadodiamide.[Pubmed:26885631]
Invest Radiol. 2016 Nov;51(11):701-705.
OBJECTIVE: The purpose of this study was to assess the incidence of nephrogenic systemic fibrosis (NSF) before and after educational interventions, implementation of a clinical screening process, and change to gadobenate dimeglumine in patients who had an estimated glomerular filtration rate (eGFR) of 30 mL/min per 1.72 m or less. METHODS: This is a Health Insurance Portability and Accountability Act compliant, institutional review board exempt study. Two periods were studied-July 2005 to June 2006, during which Gadodiamide was utilized as our magnetic resonance (MR) contrast agent, and November 2006 to August 2014, during which gadobenate dimeglumine was used as our MR contrast agent in patients who had an eGFR 30 mL/min per 1.72 m or less. In addition to a change in the MR contrast agent, education of our staff physician to the risks of NSF with MR contrast agents and the implementation of a clinical screening process occurred. The rate of NSF before and after the interventions was compared using the chi test. RESULTS: There was a statistically significant difference in the incidence of NSF in patients with an eGFR 30 mL/min per 1.72 m or less between the 2 periods: July 2005 to June 2006, 6 of 246 patients were diagnosed with NSF (P < 0.001), versus November 2006 to August 2014, 0 of 1423 patients were diagnosed with NSF. CONCLUSIONS: Our data demonstrates a marked decrease in the incidence of NSF after education of our referring physicians, implementation of clinical screening process, and change to gadobenate dimeglumine from Gadodiamide in patients with renal insufficiency. This approach potentially provides an acceptable risk-benefit profile for patients with renal insufficiency that required MR imaging for clinical care.
Moderate Renal Failure Accentuates T1 Signal Enhancement in the Deep Cerebellar Nuclei of Gadodiamide-Treated Rats.[Pubmed:28067754]
Invest Radiol. 2017 May;52(5):255-264.
OBJECTIVES: The purpose of this preclinical study was to investigate whether moderate chronic kidney disease is a factor in potentiating gadolinium (Gd) uptake in the brain. MATERIALS AND METHODS: A comparative study was performed on renally impaired (subtotal nephrectomy) rats versus rats with normal renal function. The animals received 4 daily injections of 0.6 mmol Gd/kg a week for 5 weeks (cumulative dose of 12 mmol Gd/kg) of Gadodiamide or saline solution. The MR signal enhancement in the deep cerebellar nuclei was monitored by weekly magnetic resonance imaging examinations. One week after the final injection, the total Gd concentration was determined by inductively coupled plasma mass spectrometry in different regions of the brain including the cerebellum, plasma, cerebrospinal fluid, parietal bone, and femur. RESULTS: After the administration of Gadodiamide, the subtotal nephrectomy group presented a significantly higher T1 signal enhancement in the deep cerebellar nuclei and a major increase in the total Gd concentration in all the studied structures, compared with the normal renal function group receiving the same linear Gd-based contrast agent. Those potentiated animals also showed a pronounced hypersignal in the choroid plexus, still persistent 6 days after the last injection, whereas low concentration of Gd was found in the cerebrospinal fluid (<0.05 mumol/L) at this time point. Plasma Gd concentration was then around 1 mumol/L. Interestingly, plasma Gd was predominantly in a dissociated and soluble form (around 90% of total Gd). Total Gd concentrations in the brain, cerebellum, plasma, and bones correlated with creatinine clearance in both the Gadodiamide-treated groups. CONCLUSIONS: Renal insufficiency in rats potentiates Gd uptake in the cerebellum, brain, and bones.
Contrast Agent-Induced High Signal Intensity in Dentate Nucleus on Unenhanced T1-Weighted Images: Comparison of Gadodiamide and Gadoxetic Acid.[Pubmed:28195932]
Invest Radiol. 2017 Jul;52(7):389-395.
OBJECTIVE: The aim of this study was to evaluate whether an association exists between T1-signal increase in the dentate nucleus (DN) on unenhanced magnetic resonance imaging and previous administration of gadoxetic acid and Gadodiamide. MATERIALS AND METHODS: This retrospective study was approved by the institutional review board; the requirement for informed patient consent was waived. A total of 132 patients (male-female ratio, 86:46; mean age, 68.8 +/- 11.6 years) who underwent imaging between December 2000 and April 2016 were divided into 4 groups: patients with 5 or more administrations of gadoxetic acid ("gadoxetic acid >/=5 administrations" group), only 1 administration of gadoxetic acid ("gadoxetic acid 1 administration" group), no gadolinium-based contrast agent (GBCA) administration or chronic liver disease (CLD; "no GBCA administration and no CLD" group), and 5 or more administrations of Gadodiamide ("Gadodiamide >/=5 administrations" group). Unenhanced T1-weighted images were quantitatively analyzed by 2 radiologists. Intergroup comparison of DN-to-pons signal intensity ratios was performed by the Dunn test, with the no GBCA administration and no CLD group as control. Interobserver agreement was assessed by intraclass correlation coefficients. RESULTS: The DN-to-pons ratio of the "Gadodiamide >/=5 administrations" group was significantly higher (P < 0.0001) and those of the "gadoxetic acid >/=5 administrations" and "gadoxetic acid 1 administration" groups did not differ significantly (P = 0.3912 and 1.0000, respectively) compared with the DN-to-pons ratio of the "no GBCA administration and no CLD" group. The interobserver intraclass correlation coefficient for measurement of DN-to-pons ratio was excellent (0.835; 95% confidence interval, 0.767-0.883). CONCLUSIONS: Hyperintensity in the DN on unenhanced T1-weighted images is associated with previous administration of Gadodiamide but not gadoxetic acid. Although the number of administrations for the 2 GBCA groups was identical, the administered dose of gadoxetic acid was only a quarter the amount of gadolinium as those with Gadodiamide. This difference might influence the results of this study.


