GRK2iGRK2 inhibitory polypeptide. Gβγ antagonist CAS# 148505-03-7 |
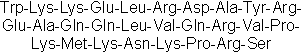
- Vilazodone
Catalog No.:BCC2040
CAS No.:163521-12-8
- SB 271046 hydrochloride
Catalog No.:BCC1924
CAS No.:209481-24-3
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- SEA0400
Catalog No.:BCC1941
CAS No.:223104-29-8
- Tianeptine
Catalog No.:BCC1999
CAS No.:66981-73-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 148505-03-7 | SDF | Download SDF |
| PubChem ID | 90488863 | Appearance | Powder |
| Formula | C153H256N50O41S | M.Wt | 3484.08 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in water | ||
| Sequence | WKKELRDAYREAQQLVQRVPKMKNKPRS | ||
| SMILES | CC(C)CC(C(=O)NC(CCCNC(=N)N)C(=O)NC(CC(=O)O)C(=O)NC(C)C(=O)NC(CC1=CC=C(C=C1)O)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCC(=O)O)C(=O)NC(C)C(=O)NC(CCC(=O)N)C(=O)NC(CCC(=O)N)C(=O)NC(CC(C)C)C(=O)NC(C(C)C)C(=O)NC(CCC(=O)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(C(C)C)C(=O)N2CCCC2C(=O)NC(CCCCN)C(=O)NC(CCSC)C(=O)NC(CCCCN)C(=O)NC(CC(=O)N)C(=O)NC(CCCCN)C(=O)N3CCCC3C(=O)NC(CCCNC(=N)N)C(=O)NC(CO)C(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(CCCCN)NC(=O)C(CCCCN)NC(=O)C(CC4=CNC5=CC=CC=C54)N | ||
| Standard InChIKey | QYWJBYYWQATYFS-ICUWLHSYSA-N | ||
| Standard InChI | InChI=1S/C153H256N50O41S/c1-78(2)70-105(195-136(230)102(51-56-118(212)213)188-127(221)91(33-15-20-59-155)181-126(220)90(32-14-19-58-154)180-124(218)88(159)73-85-76-176-89-31-13-12-30-87(85)89)140(234)184-95(38-25-64-173-151(166)167)131(225)198-109(75-119(214)215)139(233)178-83(10)123(217)194-107(72-84-43-45-86(205)46-44-84)141(235)185-94(37-24-63-172-150(164)165)129(223)187-101(50-55-117(210)211)125(219)177-82(9)122(216)179-98(47-52-113(160)206)133(227)186-99(48-53-114(161)207)135(229)196-106(71-79(3)4)143(237)200-120(80(5)6)146(240)192-100(49-54-115(162)208)134(228)183-97(40-27-66-175-153(170)171)138(232)201-121(81(7)8)148(242)203-68-29-42-112(203)145(239)190-93(35-17-22-61-157)128(222)189-103(57-69-245-11)137(231)182-92(34-16-21-60-156)130(224)197-108(74-116(163)209)142(236)193-104(36-18-23-62-158)147(241)202-67-28-41-111(202)144(238)191-96(39-26-65-174-152(168)169)132(226)199-110(77-204)149(243)244/h12-13,30-31,43-46,76,78-83,88,90-112,120-121,176,204-205H,14-29,32-42,47-75,77,154-159H2,1-11H3,(H2,160,206)(H2,161,207)(H2,162,208)(H2,163,209)(H,177,219)(H,178,233)(H,179,216)(H,180,218)(H,181,220)(H,182,231)(H,183,228)(H,184,234)(H,185,235)(H,186,227)(H,187,223)(H,188,221)(H,189,222)(H,190,239)(H,191,238)(H,192,240)(H,193,236)(H,194,217)(H,195,230)(H,196,229)(H,197,224)(H,198,225)(H,199,226)(H,200,237)(H,201,232)(H,210,211)(H,212,213)(H,214,215)(H,243,244)(H4,164,165,172)(H4,166,167,173)(H4,168,169,174)(H4,170,171,175)/t82-,83-,88-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,120-,121-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GRK2 inhibitory polypeptide that specifically inhibits Gβγ activation of GRK2. Corresponds to the Gβγ-binding domain and acts as a cellular Gβγ antagonist. |

GRK2i Dilution Calculator

GRK2i Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MNS
Catalog No.:BCC3943
CAS No.:1485-00-3
- JMV 390-1
Catalog No.:BCC5922
CAS No.:148473-36-3
- L-732,138
Catalog No.:BCC6821
CAS No.:148451-96-1
- Prion Protein 106-126 (human)
Catalog No.:BCC6027
CAS No.:148439-49-0
- (+)-Matairesinol
Catalog No.:BCN7021
CAS No.:148409-36-3
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- Secoisolariciresinol Diglucoside
Catalog No.:BCN1212
CAS No.:148244-82-0
- H-Dap-OH.HCl
Catalog No.:BCC3186
CAS No.:1482-97-9
- UNC 0642
Catalog No.:BCC8014
CAS No.:1481677-78-4
- (±)-Epibatidine
Catalog No.:BCC6750
CAS No.:148152-66-3
- trans-2-Tridecene-1,13-dioic acid
Catalog No.:BCN3667
CAS No.:14811-82-6
- Ac-Lys(Fmoc)-OH
Catalog No.:BCC2679
CAS No.:148101-51-3
- Pregabalin
Catalog No.:BCN2175
CAS No.:148553-50-8
- 3,5-Dihydroxyergosta-7,22-dien-6-one
Catalog No.:BCN1658
CAS No.:14858-07-2
- 3-O-Methylquercetin tetraacetate
Catalog No.:BCN1659
CAS No.:1486-69-7
- 3-O-Methylquercetin
Catalog No.:BCN1660
CAS No.:1486-70-0
- Fmoc-Prolinol
Catalog No.:BCC2710
CAS No.:148625-77-8
- GR 127935 hydrochloride
Catalog No.:BCC7081
CAS No.:148642-42-6
- L-733,060 hydrochloride
Catalog No.:BCC5707
CAS No.:148687-76-7
- SB 204070
Catalog No.:BCC5752
CAS No.:148688-01-1
- Tyrphostin AG 879
Catalog No.:BCC4514
CAS No.:148741-30-4
- (R)-2-Methylcysteine HCl
Catalog No.:BCC4017
CAS No.:148766-37-4
- Carboxy-PTIO, potassium salt
Catalog No.:BCC6789
CAS No.:148819-94-7
- Bismuth Subsalicylate
Catalog No.:BCC3739
CAS No.:14882-18-9
Muscarinic receptor control of pyramidal neuron membrane potential in the medial prefrontal cortex (mPFC) in rats.[Pubmed:26186898]
Neuroscience. 2015 Sep 10;303:474-88.
Damage to the cholinergic input to the prefrontal cortex has been implicated in neuropsychiatric disorders. Cholinergic endings release acetylcholine, which activates nicotinic and/or G-protein-coupled muscarinic receptors. Muscarinic receptors activate transduction systems, which control cellular effectors that regulate the membrane potential in medial prefrontal cortex (mPFC) neurons. The mechanisms responsible for the cholinergic-dependent depolarization of mPFC layer V pyramidal neurons in slices obtained from young rats were elucidated in this study. Glutamatergic and GABAergic transmission as well as tetrodotoxin (TTX)-sensitive Na(+) and voltage-dependent Ca(++) currents were eliminated. Cholinergic receptor stimulation by carbamoylcholine chloride (CCh; 100 muM) evoked depolarization (10.0 +/- 1.3 mV), which was blocked by M1/M4 (pirenzepine dihydrochloride, 2 muM) and M1 (VU 0255035, 5 muM) muscarinic receptor antagonists and was not affected by a nicotinic receptor antagonist (mecamylamine hydrochloride, 10 muM). CCh-dependent depolarization was attenuated by extra- (20 muM) or intracellular (50 muM) application of an inhibitor of the betagamma-subunit-dependent transduction system (gallein). It was also inhibited by intracellular application of a betagamma-subunit-binding peptide (GRK2i, 10muM). mPFC pyramidal neurons express Nav1.9 channels. CCh-dependent depolarization was abolished in the presence of antibodies against Nav1.9 channels in the intracellular solution and augmented by the presence of ProTx-I toxin (100 nM) in the extracellular solution. CCh-induced depolarization was not affected by the following reagents: intracellular transduction system blockers, including U-73122 (10 muM), chelerythrine chloride (5 muM), SQ 22536 (100 muM) and H-89 (2 muM); channel blockers, including Ba(++) ions (200 muM), apamin (100 nM), flufenamic acid (200 muM), 2-APB (200 muM), SKF 96365 (50 muM), and ZD 7288 (50 muM); and a Na(+)/Ca(++) exchanger blocker, benzamil (20 muM). We conclude that muscarinic M1 receptor-dependent depolarization in mPFC pyramidal neurons is evoked by the activation of Nav1.9 channels and that the signal transduction pathway involves G-protein betagamma subunits.
G-protein betagamma subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity.[Pubmed:25941381]
Proc Natl Acad Sci U S A. 2015 May 19;112(20):6497-502.
Kv7.4 channels are a crucial determinant of arterial diameter both at rest and in response to endogenous vasodilators. However, nothing is known about the factors that ensure effective activity of these channels. We report that G-protein betagamma subunits increase the amplitude and activation rate of whole-cell voltage-dependent K(+) currents sensitive to the Kv7 blocker linopirdine in HEK cells heterologously expressing Kv7.4, and in rat renal artery myocytes. In excised patch recordings, Gbetagamma subunits (2-250 ng /mL) enhanced the open probability of Kv7.4 channels without changing unitary conductance. Kv7 channel activity was also augmented by stimulation of G-protein-coupled receptors. Gallein, an inhibitor of Gbetagamma subunits, prevented these stimulatory effects. Moreover, gallein and two other structurally different Gbetagamma subunit inhibitors (GRK2i and a beta-subunit antibody) abolished Kv7 channel currents in the absence of either Gbetagamma subunit enrichment or G-protein-coupled receptor stimulation. Proximity ligation assay revealed that Kv7.4 and Gbetagamma subunits colocalized in HEK cells and renal artery smooth muscle cells. Gallein disrupted this colocalization, contracted whole renal arteries to a similar degree as the Kv7 inhibitor linopirdine, and impaired isoproterenol-induced relaxations. Furthermore, mSIRK, which disassociates Gbetagamma subunits from alpha subunits without stimulating nucleotide exchange, relaxed precontracted arteries in a linopirdine-sensitive manner. These results reveal that Gbetagamma subunits are fundamental for Kv7.4 activation and crucial for vascular Kv7 channel activity, which has major consequences for the regulation of arterial tone.
Nerve growth factor induces neurite outgrowth of PC12 cells by promoting Gbetagamma-microtubule interaction.[Pubmed:25552352]
BMC Neurosci. 2014 Dec 31;15:132.
BACKGROUND: Assembly and disassembly of microtubules (MTs) is critical for neurite outgrowth and differentiation. Evidence suggests that nerve growth factor (NGF) induces neurite outgrowth from PC12 cells by activating the receptor tyrosine kinase, TrkA. G protein-coupled receptors (GPCRs) as well as heterotrimeric G proteins are also involved in regulating neurite outgrowth. However, the possible connection between these pathways and how they might ultimately converge to regulate the assembly and organization of MTs during neurite outgrowth is not well understood. RESULTS: Here, we report that Gbetagamma, an important component of the GPCR pathway, is critical for NGF-induced neuronal differentiation of PC12 cells. We have found that NGF promoted the interaction of Gbetagamma with MTs and stimulated MT assembly. While Gbetagamma-sequestering peptide GRK2i inhibited neurite formation, disrupted MTs, and induced neurite damage, the Gbetagamma activator mSIRK stimulated neurite outgrowth, which indicates the involvement of Gbetagamma in this process. Because we have shown earlier that prenylation and subsequent methylation/demethylation of gamma subunits are required for the Gbetagamma-MTs interaction in vitro, small-molecule inhibitors (L-28 and L-23) targeting prenylated methylated protein methyl esterase (PMPMEase) were tested in the current study. We found that these inhibitors disrupted Gbetagamma and MuTau organization and affected cellular morphology and neurite outgrowth. In further support of a role of Gbetagamma-MT interaction in neuronal differentiation, it was observed that overexpression of Gbetagamma in PC12 cells induced neurite outgrowth in the absence of added NGF. Moreover, overexpressed Gbetagamma exhibited a pattern of association with MTs similar to that observed in NGF-differentiated cells. CONCLUSIONS: Altogether, our results demonstrate that betagamma subunit of heterotrimeric G proteins play a critical role in neurite outgrowth and differentiation by interacting with MTs and modulating MT rearrangement.
Two distinct mechanisms mediate acute mu-opioid receptor desensitization in native neurons.[Pubmed:19279269]
J Neurosci. 2009 Mar 11;29(10):3322-7.
Sustained stimulation of G-protein coupled receptors (GPCRs) leads to rapid loss of receptor function (acute desensitization). For many GPCRs including the mu-opioid receptor (MOR), an accepted mechanism for acute desensitization is through G-protein coupled receptor kinase (GRKs) mediated phosphorylation of the receptor, which facilitates the binding of beta-arrestins (betaarrs) to the receptor and then promotes endocytosis. However, the mechanism(s) that mediate acute desensitization have not yet been well defined in native neurons. This study used whole-cell patch clamp recording of G-protein coupled inward-rectifying potassium (GIRK) currents to assay MOR function and identify mechanisms of acute MOR desensitization in locus ceruleus (LC) neurons. The rate and extent of MOR desensitization were unaffected by beta(arr)-2 knock-out. Disruption of GRK2 function via inhibitory peptide introduced directly into neurons also failed to affect desensitization in wild type or beta(arr)-2 knock-outs. Inhibition of ERK1/2 activation alone had little effect on acute desensitization. However, when both GRK2-beta(arr)-2 and ERK1/2 functions were disrupted simultaneously, desensitization of MOR was nearly abolished. Together, these results suggest that acute desensitization of MOR in native LC neurons is determined by at least two molecular pathways, one involving GRK2 and beta(arr)2, and a parallel pathway mediated by activated ERK1/2.
A betagamma dimer derived from G13 transduces the angiotensin AT1 receptor signal to stimulation of Ca2+ channels in rat portal vein myocytes.[Pubmed:9287322]
J Biol Chem. 1997 Sep 12;272(37):23180-5.
A G protein composed of alpha13, beta1, and gamma3 subunits selectively couples the angiotensin AT1A receptors to increase cytoplasmic Ca2+ concentration ([Ca2+]i) in rat portal vein myocytes (Macrez-Lepretre, N., Kalkbrenner, F., Morel, J. L., Schultz, G., and Mironneau, J. (1997) J. Biol. Chem. 272, 10095-10102). We show here that Gbetagamma transduces the signal leading to stimulation of L-type Ca2+ channels. Intracellular dialysis through the patch pipette of a carboxyl-terminal anti-betacom antibody and a peptide corresponding to the Gbetagamma binding region of the beta-adrenergic receptor kinase 1 inhibited the stimulation of Ca2+ channels and the increase in [Ca2+]i evoked by angiotensin II. The Gbetagamma binding peptide did not prevent the dissociation of the heterotrimeric G protein into its subunits, as it did not block activation of phospholipase C-beta by Galphaq in response to stimulation of alpha1-adrenoreceptors. Transient overexpression of the beta-adrenergic receptor kinase 1 fragment and of Galpha subunits also inhibited the angiotensin II-induced increase in [Ca2+]i. Both anti-alpha13 antibody and carboxyl-terminal alpha13 peptide abrogated the angiotensin II-induced stimulation of Ca2+ channels. We conclude that activation of angiotensin AT1 receptors requires all three alpha, beta, and gamma subunits of G13 for receptor-G protein interaction, whereas the transduction of the signal to L-type Ca2+ channels is mediated by Gbetagamma.
Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling.[Pubmed:8119963]
J Biol Chem. 1994 Feb 25;269(8):6193-7.
The beta gamma subunits (G beta gamma) of heterotrimeric G proteins modulate the activity of several signal-transducing effector molecules including G protein-coupled receptor kinases. G beta gamma binds to the carboxyl terminus of the beta-adrenergic receptor kinase (beta ARK) and regulates its activity. To investigate the effect of such a G beta gamma-binding domain on heterologous G beta gamma interactions, various receptors that can stimulate phospholipase C and/or type II adenylate cyclase were coexpressed in COS-7 cells with the carboxyl terminus of beta ARK1. Phosphoinositol hydrolysis in response to activation of receptors that stimulate phospholipase C via Gi beta gamma (alpha 2-adrenergic and M2-muscarinic cholinergic receptors) was markedly inhibited by the coexpressed beta ARK1 polypeptide, whereas that mediated by Gq alpha subunits (alpha 1-adrenergic and M1-muscarinic cholinergic receptors) was unaffected. Increased cellular cAMP levels due to stimulation of receptors and coexpressed adenylate cyclase II displayed marked inhibition in the presence of the beta ARK1 polypeptide. Moreover, inhibition of adenylate cyclase produced by alpha 2-adrenergic receptor stimulation (a Gi alpha-mediated process) was unaffected, indicating that the beta ARK1 polypeptide provides a useful tool for distinguishing between G alpha and G beta gamma pathways.


