FuzitineCAS# 142287-96-5 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 142287-96-5 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
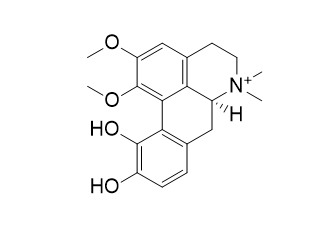
| Formula | C20H24NO4 | M.Wt | 342.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fuzitine Dilution Calculator

Fuzitine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9206 mL | 14.6028 mL | 29.2056 mL | 58.4112 mL | 73.014 mL |
| 5 mM | 0.5841 mL | 2.9206 mL | 5.8411 mL | 11.6822 mL | 14.6028 mL |
| 10 mM | 0.2921 mL | 1.4603 mL | 2.9206 mL | 5.8411 mL | 7.3014 mL |
| 50 mM | 0.0584 mL | 0.2921 mL | 0.5841 mL | 1.1682 mL | 1.4603 mL |
| 100 mM | 0.0292 mL | 0.146 mL | 0.2921 mL | 0.5841 mL | 0.7301 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Euphorbia factor L25
Catalog No.:BCN0254
CAS No.:303174-98-3
- Peiioside B
Catalog No.:BCN0253
CAS No.:1610618-91-1
- Gynosaponin TN2
Catalog No.:BCN0252
CAS No.:77658-95-8
- 4'-O-Methyllucenin II (Diosmetin 6,8-di-C-glucoside)
Catalog No.:BCN0251
CAS No.:98813-28-6
- Dioscoreside E
Catalog No.:BCN0250
CAS No.:435321-73-6
- Dioscoreside C
Catalog No.:BCN0249
CAS No.:344912-80-7
- O-Acetylgalanthamine
Catalog No.:BCN0248
CAS No.:25650-83-3
- Galanthamine 10-Oxide
Catalog No.:BCN0247
CAS No.:134332-50-6
- Anhydrobyankangelicin
Catalog No.:BCN0246
CAS No.:35214-81-4
- O-Desmethyl galanthamine
Catalog No.:BCN0245
CAS No.:60755-80-8
- Demethylisoencecalin
Catalog No.:BCN0244
CAS No.:24672-84-2
- 3'-Hydroxyxanthyletin
Catalog No.:BCN0243
CAS No.:165900-08-3
- Secotubeimoside I
Catalog No.:BCN0256
CAS No.:106235-32-9
- Planispine A
Catalog No.:BCN0257
CAS No.:1202761-42-9
- Euphorbia factor L22
Catalog No.:BCN0258
CAS No.:1613700-09-6
- Deoxy euphorbia factor L1
Catalog No.:BCN0259
CAS No.:247099-01-0
- Belamcandol B
Catalog No.:BCN0260
CAS No.:137786-94-8
- (Z)-5-(Heptadec-10-en-1-yl)resorcinol
Catalog No.:BCN0261
CAS No.:52483-21-3
- Wilfoside K 1N
Catalog No.:BCN0262
CAS No.:100802-91-3
- Bungeiside B
Catalog No.:BCN0263
CAS No.:149561-88-6
- Wilfoside C 1N
Catalog No.:BCN0264
CAS No.:96829-53-7
- Cynandione A
Catalog No.:BCN0265
CAS No.:168706-29-4
- (-)-Conduritol F
Catalog No.:BCN0266
CAS No.:6090-98-8
- Eleutheroside B1
Catalog No.:BCN0267
CAS No.:16845-16-2
Investigation of the chemical markers for experiential quality evaluation of crude aconite by UHPLC-Q-TOF-MS.[Pubmed:27624993]
J Sep Sci. 2016 Nov;39(22):4281-4289.
Many foods and herbs are experientially classified into different commodity grades in commercial circulation. Regarding the hypertoxic herb aconite, large samples are considered to be of better quality. However, this experiential classification lacks a scientific basis. In this study, we focused on the quality diversity among different grades and studied it using the minimum lethal dose assay and a novel ultra high performance liquid chromatography coupled with time-of-flight mass spectrometry method. Toxicity assay result suggested grade I aconite had the lowest toxicity (p < 0.05). Using this method with partial least squares-discriminant analysis, we discovered nine chemomarkers, including neoline, songorine, fuziline, mesaconitine, talatizidine, dexyaconitine, talatisamine, hypaconitine, and Fuzitine. Considering their toxicity and activity, we found the levels of toxic ingredients hypaconitine, dexyaconitine, and mesaconitine in grade I were lower than those in grade II (p < 0.01), while the levels of efficacy ingredients songorine, talatisamine, and neoline were the highest in grade I (p < 0.01). Further study demonstrated that the quality variation was associated with plant tissue development and toxic ingredient distribution law. Our results provide scientific evidence for the experiential quality evaluation of aconite, and it will be of great utility for other foods and herbs.
New feruloyl tyramine glycosides from Stephania hispidula YAMAMOTO.[Pubmed:20190454]
Chem Pharm Bull (Tokyo). 2010 Mar;58(3):415-7.
Three new feruloyl tyramine glycosides, N-cis-feruloyl tyramine-4'''-O-beta-D-glucopyranoside (1), N-trans-ferloyl tyramine-4'''-O-beta-D-glucopyranoside (2), and N-trans-feruloyl tyramine-4'-O-beta-D-glucopyranoside (3), along with six known compounds, N-trans-feruloyl-3'''-methoxydopamine-4'-O-beta-D-glucopyranoside (4), haitinosporine (5), tubocurine (6), Fuzitine (7), (+)-lyoniresinol-3alpha-O-beta-D-glucopyranoside (8), and (-)-lyoniresinol-2alpha-O-beta-D-glucopyranoside (9), were isolated from the stem of Stephania hispidula YAMAMOTO. The structures were elucidated by spectroscopic and chemical analysis.
A new alkaloid and its artificial derivative with an indazole ring from Nigella glandulifera.[Pubmed:15056964]
Chem Pharm Bull (Tokyo). 2004 Apr;52(4):454-5.
A new compound, nigeglanine (1), and its new artificial derivative (1a), were isolated from the seeds of Nigella glandulifera, together with a known aporphine alkaloid, Fuzitine (2). Their structures were established by spectral analysis, including two-dimensional (2D)-NMR spectroscopy. Nigeglanine (1) is the third natural product determined to contain an indazole nucleus.
[Studies on the alkaloid constituents of Jiangyou fu-zi Aconitum carmichaeli from Sichuan].[Pubmed:1293938]
Yao Xue Xue Bao. 1992;27(9):670-3.
Six compounds were isolated from the aqueous extract of Aconitum carmichaeli Debx (cultivated in Jiang-you region of Sichuan province). Five of them have been identified as uracil (I), songorine HCl(II), karakoline(III), neoline(IV), and Fuzitine(VI). Compound V is a new C19-diterpenoid alkaloid determined as C33H47NO9 and named neojiangyouaconitine.


