FraxinCAS# 524-30-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 524-30-1 | SDF | Download SDF |
| PubChem ID | 5281418 | Appearance | White powder |
| Formula | C16H18O10 | M.Wt | 370.32 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | 7,8-Dihydroxy 6-methoxycoumarin 8-β-D-glucopyranoside; Fraxetin 8-β-D-glucopyranoside; Fraxetol 8-glucoside; Fraxoside | ||
| Solubility | DMSO : 250 mg/mL (675.11 mM; Need ultrasonic) | ||
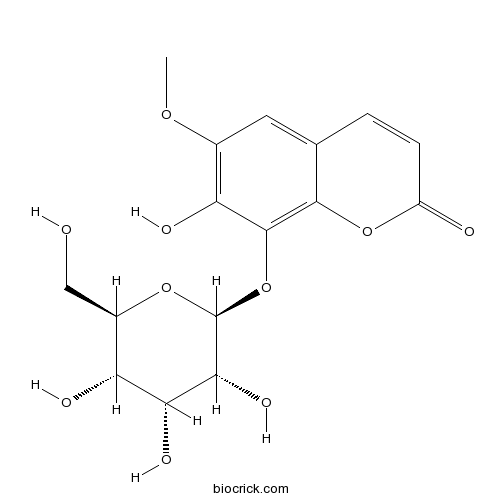
| Chemical Name | 7-hydroxy-6-methoxy-8-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-2-one | ||
| SMILES | COC1=C(C(=C2C(=C1)C=CC(=O)O2)OC3C(C(C(C(O3)CO)O)O)O)O | ||
| Standard InChIKey | CRSFLLTWRCYNNX-YJDQBUFVSA-N | ||
| Standard InChI | InChI=1S/C16H18O10/c1-23-7-4-6-2-3-9(18)25-14(6)15(11(7)20)26-16-13(22)12(21)10(19)8(5-17)24-16/h2-4,8,10,12-13,16-17,19-22H,5H2,1H3/t8-,10-,12-,13-,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fraxin possesses a variety of bioactivities such as anti-inflammatory, antioxidant, analgesic, antimicrobial, antiviral, immunomodulatory, anti-hyperuricemia and diuresis. Fraxin enhances urate excretion partly by inhibiting mURAT1 or mGLUT9 in kidney of hyperuricemic mice. |
| Targets | GLUT | mURAT1 | mOAT1 | mOCT1 |
| In vitro | Natural compounds,fraxin and chemicals structurally related to fraxin protect cells from oxidative stress.[Pubmed: 16264268]Exp Mol Med. 2005 Oct 31;37(5):436-46.Coumarins comprise a group of natural phenolic compounds found in a variety of plant sources. In view of the established low toxicity, relative cheapness, presence in the diet and occurrence in various herbal remedies of coumarins, it appears prudent to evaluate their properties and applications further. |
| In vivo | Metabolic fate of fraxin administered orally to rats.[Pubmed: 16724835]J Nat Prod. 2006 May;69(5):755-7.
Protective effects of cortex fraxini coumarines against oxonate-induced hyperuricemia and renal dysfunction in mice.[Pubmed: 21620826]Eur J Pharmacol. 2011 Sep;666(1-3):196-204.The aim of the present study was to investigate the effects of cortex Fraxini coumarines esculetin, esculin, fraxetin and Fraxin on renal dysfunction and expression abnormality of renal organic ion transporters in hyperuricemic animals. |
| Structure Identification | Biomed Chromatogr. 2005 Nov;19(9):696-702.Non-aqueous capillary electrophoresis for separation and simultaneous determination of fraxin, esculin and esculetin in Cortex fraxini and its medicinal preparations.[Pubmed: 15828063]
|

Fraxin Dilution Calculator

Fraxin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7004 mL | 13.5018 mL | 27.0037 mL | 54.0073 mL | 67.5092 mL |
| 5 mM | 0.5401 mL | 2.7004 mL | 5.4007 mL | 10.8015 mL | 13.5018 mL |
| 10 mM | 0.27 mL | 1.3502 mL | 2.7004 mL | 5.4007 mL | 6.7509 mL |
| 50 mM | 0.054 mL | 0.27 mL | 0.5401 mL | 1.0801 mL | 1.3502 mL |
| 100 mM | 0.027 mL | 0.135 mL | 0.27 mL | 0.5401 mL | 0.6751 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dauricine
Catalog No.:BCN4977
CAS No.:524-17-4
- gamma-Fagarine
Catalog No.:BCN5673
CAS No.:524-15-2
- Wedelolactone
Catalog No.:BCN5672
CAS No.:524-12-9
- Epistephamiersine
Catalog No.:BCN5671
CAS No.:52389-15-8
- Erysotramidine
Catalog No.:BCN5670
CAS No.:52358-58-4
- 2-Benzoyl-1,3,4,4a,5,8a-hexahydro-6(2H)-isoquinolinone
Catalog No.:BCC8559
CAS No.:52346-14-2
- Alnusone
Catalog No.:BCN8108
CAS No.:52330-11-7
- 6alpha-Chloro-5beta-hydroxywithaferin A
Catalog No.:BCN8007
CAS No.:52329-20-1
- Dimethylcurcumin
Catalog No.:BCN2748
CAS No.:52328-98-0
- Tetramethylcurcumin
Catalog No.:BCN2746
CAS No.:52328-97-9
- p,p-hydroxy-curucumin
Catalog No.:BCC8890
CAS No.:52328-96-8
- Vindorosine
Catalog No.:BCN5668
CAS No.:5231-60-7
- 2-APB
Catalog No.:BCC6978
CAS No.:524-95-8
- Noricaritin
Catalog No.:BCN5353
CAS No.:5240-95-9
- (R)-DPN
Catalog No.:BCC7939
CAS No.:524047-78-7
- H-Phe-NH2
Catalog No.:BCC3008
CAS No.:5241-58-7
- Boc-D-Trp-OH
Catalog No.:BCC3457
CAS No.:5241-64-5
- Boc-D-Met-OH
Catalog No.:BCC3426
CAS No.:5241-66-7
- Cochlearine
Catalog No.:BCN1929
CAS No.:52418-07-2
- Oxeladin Citrate
Catalog No.:BCC3831
CAS No.:52432-72-1
- Isobutylshikonin
Catalog No.:BCN3005
CAS No.:52438-12-7
- 1-Cinnamoylpyrrolidine
Catalog No.:BCN4086
CAS No.:52438-21-8
- ERB 041
Catalog No.:BCC7903
CAS No.:524684-52-4
- Vandetanib hydrochloride
Catalog No.:BCC2028
CAS No.:524722-52-9
Metabolic fate of fraxin administered orally to rats.[Pubmed:16724835]
J Nat Prod. 2006 May;69(5):755-7.
Naturally occurring Fraxin (1) was administered orally to rats to investigate its metabolism. Urinary metabolites were analyzed by three-dimensional HPLC, and fraxetin-7-O-sulfate (2), fraxetin-7-O-beta-glucuronide (3), fraxetin (4), 6,7,8-trihydroxycoumarin (5), and fraxidin (6) were isolated. Fraxin (1) was extensively metabolized to 4, which was partly metabolized to 5 in a rat fecal suspension after incubation for 24 h. Urinary excretion of 4 and 5 in rats administered orally with 1 was substantially reduced when the rats were treated with antibiotics to suppress their intestinal flora. Incubation of 1 with a rat liver S-9 mixture yielded 6. These results suggest that hydrolysis and demethylation of 1 are performed by intestinal microflora, while methylation occurs in the liver.
Non-aqueous capillary electrophoresis for separation and simultaneous determination of fraxin, esculin and esculetin in Cortex fraxini and its medicinal preparations.[Pubmed:15828063]
Biomed Chromatogr. 2005 Nov;19(9):696-702.
A non-aqueous capillary electrophoresis method has been developed for the separation and simultaneous determination of Fraxin, esculin and esculetin in Cortex Fraxini and its preparation for the first time. Optimum separation of the analytes was obtained on a 47 cm x 75 microm i.d. fused-silica capillary using a non-aqueous buffer system of 60 mM sodium cholate, 20 mM ammonium acetate, 20% acetonitrile and 3% acetic acid at 20 kV and 292 K, respectively. The relative standard deviations (RSDs) of the migration times and the peak heights of the three analytes were in the range of 0.23-0.28 and 2.12-2.60%, respectively. Detection limits of Fraxin, esculin and esculetin were 0.1557, 0.4073 and 0.5382 microg/mL, respectively. In the tested concentration range, good linear relationships (correlation coefficients 0.9995 for Fraxin, 0.9999 for esculin and 0.9992 for esculetin) between peak heights and concentrations of the analytes were observed. This method has been successfully applied to simultaneous determination of the three bioactive components with the recoveries from 90.2 to 109.2% in the five samples.
Protective effects of cortex fraxini coumarines against oxonate-induced hyperuricemia and renal dysfunction in mice.[Pubmed:21620826]
Eur J Pharmacol. 2011 Sep;666(1-3):196-204.
The aim of the present study was to investigate the effects of cortex Fraxini coumarines esculetin, esculin, fraxetin and Fraxin on renal dysfunction and expression abnormality of renal organic ion transporters in hyperuricemic animals. Mice were orally given 250 mg/kg oxonate for seven consecutive days to induce hyperuricemia and renal dysfunction. After 1h of oxonate induction daily, animals were orally treated with esculetin, esculin, fraxetin and Fraxin at 20 and 40 mg/kg, respectively. Esculetin, esculin, fraxetin and Fraxin significantly decreased serum urate, creatinine and blood urea nitrogen levels and increased urine urate and creatinine excretion in hyperuricemic mice. Esculetin and esculin up-regulated expressions of renal organic anion transporter 1 (mOAT1), organic cation and carnitine transporters (mOCT1-2 and mOCTN1-2), but failed to affect renal glucose transporter 9 (mGLUT9) and urate transporter 1 (mURAT1) in this model. Fraxetin specifically inhibited renal mURAT1, while Fraxin extensively interacted with renal mGLUT9, mURAT1, mOAT1 and mOCT1 in hyperuricemic mice. Furthermore, esculetin, fraxetin and Fraxin increased mABCG2 mRNA expression and decreased its protein levels in renal apical membrane in hyperuricemic mice. These results indicate that esculetin and esculin have beneficial effects on hyperuricemia and renal dysfunction, resulting in restoration of mOAT1, mOCT1-2 and mOCTN1-2, and fraxetin and Fraxin enhance urate excretion partly by inhibiting mURAT1 or mGLUT9 in kidney of hyperuricemic mice. Regulation of mABCG2 by cortex Fraxini coumarines may be partly contributed to their beneficial actions. This study provides an evidence to support clinical therapeutic effects of cortex Fraxini coumarines on hyperuricemia with renal dysfunction.


