FerruginolCAS# 514-62-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 514-62-5 | SDF | Download SDF |
| PubChem ID | 442027 | Appearance | Powder |
| Formula | C20H30O | M.Wt | 286.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
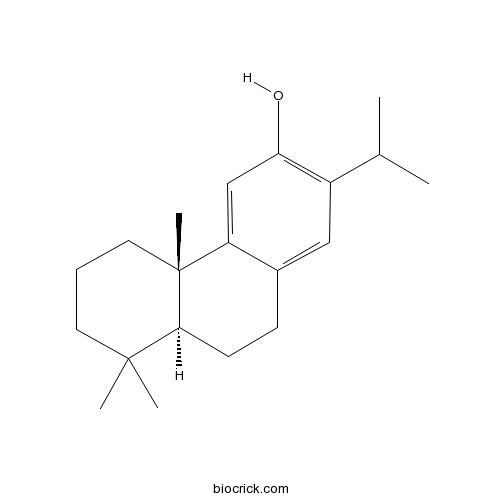
| Chemical Name | (4bS,8aS)-4b,8,8-trimethyl-2-propan-2-yl-5,6,7,8a,9,10-hexahydrophenanthren-3-ol | ||
| SMILES | CC(C)C1=C(C=C2C(=C1)CCC3C2(CCCC3(C)C)C)O | ||
| Standard InChIKey | QXNWVJOHUAQHLM-AZUAARDMSA-N | ||
| Standard InChI | InChI=1S/C20H30O/c1-13(2)15-11-14-7-8-18-19(3,4)9-6-10-20(18,5)16(14)12-17(15)21/h11-13,18,21H,6-10H2,1-5H3/t18-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Ferruginol has anti-plasmodial, leishmanicidal, anti-ulcerogenic activities. 2. Ferruginol has cardioprotective, anti-oxidative and anti-inflammatory activities. 3. Ferruginol has anticancer activity, can induce apoptosis in non-small cell lung cancer (NSCLC) cells. 4. Ferruginol has antimicrobial and antifungal activities against wood-rot fungi (basidiomycetes), can cause cellular dysfunction and damage, lead to growth inhibition and autophagic cell death of fungi. |
| Targets | PARP | Bcl-2/Bax | Caspase | Antifection |

Ferruginol Dilution Calculator

Ferruginol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4904 mL | 17.452 mL | 34.904 mL | 69.808 mL | 87.26 mL |
| 5 mM | 0.6981 mL | 3.4904 mL | 6.9808 mL | 13.9616 mL | 17.452 mL |
| 10 mM | 0.349 mL | 1.7452 mL | 3.4904 mL | 6.9808 mL | 8.726 mL |
| 50 mM | 0.0698 mL | 0.349 mL | 0.6981 mL | 1.3962 mL | 1.7452 mL |
| 100 mM | 0.0349 mL | 0.1745 mL | 0.349 mL | 0.6981 mL | 0.8726 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Euphol
Catalog No.:BCN7790
CAS No.:514-47-6
- Tirucallol
Catalog No.:BCN7787
CAS No.:514-46-5
- Parkeol
Catalog No.:BCN3728
CAS No.:514-45-4
- Periplogenin
Catalog No.:BCN2656
CAS No.:514-39-6
- Abietic acid
Catalog No.:BCN2728
CAS No.:514-10-3
- Taraxerone
Catalog No.:BCN5636
CAS No.:514-07-8
- 2-(2-Aminoethyl)-1-methylpyrrolidine
Catalog No.:BCC8477
CAS No.:51387-90-7
- Boc-Methioninol
Catalog No.:BCC2720
CAS No.:51372-93-1
- Tris DBA
Catalog No.:BCC7685
CAS No.:51364-51-3
- delta-Amyrin acetate
Catalog No.:BCN5635
CAS No.:51361-60-5
- Budesonide
Catalog No.:BCC4767
CAS No.:51333-22-3
- Soyasaponin Bb
Catalog No.:BCN2598
CAS No.:51330-27-9
- Biperiden
Catalog No.:BCC4274
CAS No.:514-65-8
- Canthaxanthin
Catalog No.:BCC8139
CAS No.:514-78-3
- Alrestatin
Catalog No.:BCC6663
CAS No.:51411-04-2
- Chikusetsusaponin IVa
Catalog No.:BCN3432
CAS No.:51415-02-2
- Odonicin
Catalog No.:BCN5637
CAS No.:51419-51-3
- 3-Methyladenine
Catalog No.:BCC3714
CAS No.:5142-23-4
- COG 133
Catalog No.:BCC1047
CAS No.:514200-66-9
- Cyclo(Tyr-Phe)
Catalog No.:BCN2423
CAS No.:5147-17-1
- Deoxynivalenol
Catalog No.:BCC7832
CAS No.:51481-10-8
- Cimetidine
Catalog No.:BCC4527
CAS No.:51481-61-9
- PMX 205
Catalog No.:BCC8039
CAS No.:514814-49-4
- H-Tyr(tBu)-OMe.HCl
Catalog No.:BCC2672
CAS No.:51482-39-4
Proteomics investigation reveals cell death-associated proteins of basidiomycete fungus Trametes versicolor treated with Ferruginol.[Pubmed:25485628]
J Agric Food Chem. 2015 Jan 14;63(1):85-91.
Ferruginol has antifungal activity against wood-rot fungi (basidiomycetes). However, specific research on the antifungal mechanisms of Ferruginol is scarce. Two-dimensional gel electrophoresis and fluorescent image analysis were employed to evaluate the differential protein expression of wood-rot fungus Trametes versicolor treated with or without Ferruginol. Results from protein identification of tryptic peptides via liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) analyses revealed 17 protein assignments with differential expression. Downregulation of cytoskeleton beta-tubulin 3 indicates that Ferruginol has potential to be used as a microtubule-disrupting agent. Downregulation of major facilitator superfamily (MFS)-multiple drug resistance (MDR) transporter and peroxiredoxin TSA1 were observed, suggesting reduction in self-defensive capabilities of T. versicolor. In addition, the proteins involved in polypeptide sorting and DNA repair were also downregulated, while heat shock proteins and autophagy-related protein 7 were upregulated. These observations reveal that such cellular dysfunction and damage caused by Ferruginol lead to growth inhibition and autophagic cell death of fungi.
Antimalarial activity of abietane ferruginol analogues possessing a phthalimide group.[Pubmed:25316317]
Bioorg Med Chem Lett. 2014 Nov 15;24(22):5234-7.
The abietane-type diterpenoid (+)-Ferruginol, a bioactive compound isolated from New Zealand's Miro tree (Podocarpus ferruginea), displays relevant pharmacological properties, including antimicrobial, cardioprotective, anti-oxidative, anti-plasmodial, leishmanicidal, anti-ulcerogenic, anti-inflammatory and anticancer. Herein, we demonstrate that Ferruginol (1) and some phthalimide containing analogues 2-12 have potential antimalarial activity. The compounds were evaluated against malaria strains 3D7 and K1, and cytotoxicity was measured against a mammalian cell line panel. A promising lead, compound 3, showed potent activity with an EC50 = 86 nM (3D7 strain), 201 nM (K1 strain) and low cytotoxicity in mammalian cells (SI>290). Some structure-activity relationships have been identified for the antimalarial activity in these abietane analogues.
The accumulation pattern of ferruginol in the heartwood-forming Cryptomeria japonica xylem as determined by time-of-flight secondary ion mass spectrometry and quantity analysis.[Pubmed:24651372]
Ann Bot. 2014 May;113(6):1029-36.
BACKGROUND AND AIMS: Heartwood formation is a unique phenomenon of tree species. Although the accumulation of heartwood substances is a well-known feature of the process, the accumulation mechanism remains unclear. The aim of this study was to determine the accumulation process of Ferruginol, a predominant heartwood substance of Cryptomeria japonica, in heartwood-forming xylem. METHODS: The radial accumulation pattern of Ferruginol was examined from sapwood and through the intermediate wood to the heartwood by direct mapping using time-of-flight secondary ion mass spectrometry (TOF-SIMS). The data were compared with quantitative results obtained from a novel method of gas chromatography analysis using laser microdissection sampling and with water distribution obtained from cryo-scanning electron microscopy. KEY RESULTS: Ferruginol initially accumulated in the middle of the intermediate wood, in the earlywood near the annual ring boundary. It accumulated throughout the entire earlywood in the inner intermediate wood, and in both the earlywood and the latewood in the heartwood. The process of Ferruginol accumulation continued for more than eight annual rings. Ferruginol concentration peaked at the border between the intermediate wood and heartwood, while the concentration was less in the latewood compared with the earlywood in each annual ring. Ferruginol tended to accumulate around the ray parenchyma cells. In addition, at the border between the intermediate wood and heartwood, the accumulation was higher in areas without water than in areas with water. CONCLUSIONS: TOF-SIMS clearly revealed Ferruginol distribution at the cellular level. Ferruginol accumulation begins in the middle of intermediate wood, initially in the earlywood near the annual ring boundary, then throughout the entire earlywood, and finally across to the whole annual ring in the heartwood. The heterogeneous timing of Ferruginol accumulation could be related to the distribution of ray parenchyma cells and/or water in the heartwood-forming xylem.
Ferruginol inhibits non-small cell lung cancer growth by inducing caspase-associated apoptosis.[Pubmed:25355727]
Integr Cancer Ther. 2015 Jan;14(1):86-97.
PURPOSE: The anti-lung cancer effect of Cryptomeria japonica leaf extractive and its active phytocompound was evaluated using in vitro and in vivo assays. EXPERIMENTAL DESIGN: The anti-lung cancer mechanism was investigated using flow cytometry and western blot analyses, and the antitumor activity was evaluated in a xenograft animal model. RESULTS: MTT assay indicated that the cytotoxic effects of Ferruginol in A549 and CL1-5 cells were dose-dependent. According to the results of cell cycle and annexin V/PI analyses, the sub-G1 population and annexin V binding in the 2 cell lines were increased after Ferruginol treatment. The results of western blot analyses revealed that the cleaved forms of caspase 3, 8, 9, and poly(ADP-ribose) polymerase were activated after Ferruginol treatment in A549 and CL1-5 cells. Moreover, the expression of the anti-apoptotic protein Bcl-2 was decreased, while the expression of the pro-apoptotic protein Bax was elevated, after Ferruginol treatment in both lung cancer cell lines. These results indicated that Ferruginol acted via a caspase-dependent mitochondrial apoptotic pathway in the 2 cell lines. Intraperitoneal administration of Ferruginol significantly suppressed the growth of subcutaneous CL1-5 xenografts. CONCLUSIONS: The findings of the present study provided insight into the molecular mechanisms underlying Ferruginol-induced apoptosis in non-small cell lung cancer (NSCLC) cells, indicating that this compound may be a potential candidate drug for anti-NSCLC.


