FGIN-1-43Potent, specific ligand for mitochondrial DBI receptor CAS# 145040-29-5 |
- BAY 87-2243
Catalog No.:BCC4131
CAS No.:1227158-85-1
- PX 12
Catalog No.:BCC2436
CAS No.:141400-58-0
- DMOG
Catalog No.:BCC2433
CAS No.:89464-63-1
- KC7F2
Catalog No.:BCC2434
CAS No.:927822-86-4
- IOX2(Glycine)
Catalog No.:BCC2229
CAS No.:931398-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 145040-29-5 | SDF | Download SDF |
| PubChem ID | 3995234 | Appearance | Powder |
| Formula | C28H36Cl2N2O | M.Wt | 487.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
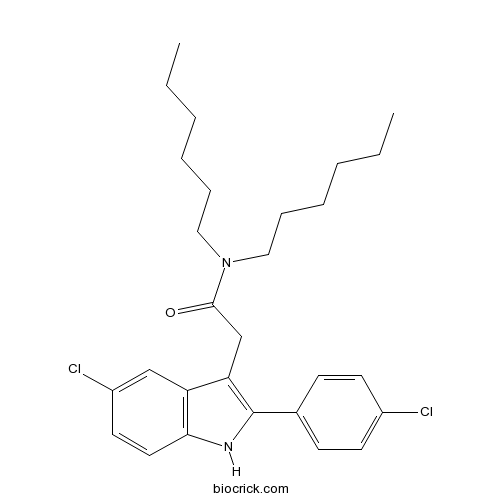
| Chemical Name | 2-[5-chloro-2-(4-chlorophenyl)-1H-indol-3-yl]-N,N-dihexylacetamide | ||
| SMILES | CCCCCCN(CCCCCC)C(=O)CC1=C(NC2=C1C=C(C=C2)Cl)C3=CC=C(C=C3)Cl | ||
| Standard InChIKey | XTZUPNNVXIMWAR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H36Cl2N2O/c1-3-5-7-9-17-32(18-10-8-6-4-2)27(33)20-25-24-19-23(30)15-16-26(24)31-28(25)21-11-13-22(29)14-12-21/h11-16,19,31H,3-10,17-18,20H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Most active of the 2-aryl-3-indoleacetamides; a new class of potent and specific ligands as probes for the mitochondial DBI receptor (peripheral benzodiazepine receptor). |

FGIN-1-43 Dilution Calculator

FGIN-1-43 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0512 mL | 10.2562 mL | 20.5124 mL | 41.0248 mL | 51.281 mL |
| 5 mM | 0.4102 mL | 2.0512 mL | 4.1025 mL | 8.205 mL | 10.2562 mL |
| 10 mM | 0.2051 mL | 1.0256 mL | 2.0512 mL | 4.1025 mL | 5.1281 mL |
| 50 mM | 0.041 mL | 0.2051 mL | 0.4102 mL | 0.8205 mL | 1.0256 mL |
| 100 mM | 0.0205 mL | 0.1026 mL | 0.2051 mL | 0.4102 mL | 0.5128 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fmoc-Asp(OMe)-OH
Catalog No.:BCC3090
CAS No.:145038-53-5
- Digalactosyldiacylglycerol
Catalog No.:BCC8941
CAS No.:145033-48-3
- ω-Agatoxin IVA
Catalog No.:BCC7488
CAS No.:145017-83-0
- Pregnenolone
Catalog No.:BCN6255
CAS No.:145-13-1
- DGAT1-IN-1
Catalog No.:BCC5511
CAS No.:1449779-49-0
- DDR1-IN-1
Catalog No.:BCC5170
CAS No.:1449685-96-4
- Boeravinone O
Catalog No.:BCN6693
CAS No.:1449384-21-7
- Chlojaponilactone B
Catalog No.:BCN7400
CAS No.:1449382-91-5
- K145 hydrochloride
Catalog No.:BCC4072
CAS No.:1449240-68-9
- Senexin B
Catalog No.:BCC3990
CAS No.:1449228-40-3
- Androst-5-en-3-ol-7,17-dione acetate
Catalog No.:BCC8822
CAS No.:1449-61-2
- 24-Methylenecycloartan-3-ol
Catalog No.:BCN6254
CAS No.:1449-09-8
- Candesartan cilexetil
Catalog No.:BCC8900
CAS No.:145040-37-5
- CPI-169
Catalog No.:BCC5396
CAS No.:1450655-76-1
- Rink Amide Linker
Catalog No.:BCC2833
CAS No.:145069-56-3
- YM 022
Catalog No.:BCC7052
CAS No.:145084-28-2
- Filicenol B
Catalog No.:BCN6446
CAS No.:145103-37-3
- Piribedil dihydrochloride
Catalog No.:BCC6898
CAS No.:1451048-94-4
- Dexmedetomidine HCl
Catalog No.:BCC4347
CAS No.:145108-58-3
- 8-Hydroxy-PIPAT oxalate
Catalog No.:BCC6800
CAS No.:1451210-48-2
- 6-Hydroxykaempferol-3,6,7-triglucoside
Catalog No.:BCN1562
CAS No.:145134-62-9
- 6-Hydroxykaempferol 3-Rutinoside -6-glucoside
Catalog No.:BCN1561
CAS No.:145134-63-0
- CP 99994 dihydrochloride
Catalog No.:BCC6016
CAS No.:145148-39-6
- 3beta-Hydroxyporiferast-5-en-7-one
Catalog No.:BCN6256
CAS No.:145163-97-9
Synthesis and preliminary behavioural evaluation in mice of new 3-aryl-3-pyrrol-1-ylpropanamides, analogues of FGIN-1-27 and FGIN-1-43.[Pubmed:11732760]
J Pharm Pharmacol. 2001 Nov;53(11):1561-8.
The 2-aryl-3-indoleacetamides FGIN-1-27 and FGIN-1-43 have already been characterized in-vitro as potent and specific ligands for the mitochondrial DBI receptor. This affinity was associated with psychotropic properties in several rodent behavioural tasks (in particular anxiolytic action) via enhancement of GABA transmission through neurosteroid production. The synthesis of new 3-aryl-3-pyrrol-1-ylpropanamides 1a-i, analogues of FGIN-1-27 and FGIN-1-43, is described in four steps starting from the corresponding arylaldehydes. Preliminary evaluation of these compounds in behavioural studies (spontaneous locomotor activity and anxiolytic activity) in mice was also undertaken.
Chemistry, binding affinities, and behavioral properties of a new class of "antineophobic" mitochondrial DBI receptor complex (mDRC) ligands.[Pubmed:8411007]
J Med Chem. 1993 Oct 1;36(20):2908-20.
The mitochondrial DBI receptor complex (mDRC; previously called the peripheral benzodiazepine receptors) is linked to the production of neurosteroids such as pregnenolone sulfate, dehydroepiandrosterone sulfate, and others. In order to gain further information as to the function of the mDRC in the brain, we have constructed and tested both in vitro and in vivo a novel series of ligands, 2-arylindole-3-acetamides. The SAR studies detailed herein delineate some of the structural features required for high affinity binding to the mDRCs. In most cases the new ligands were prepared by use of the Fischer indole synthesis. Variations in the length and number of the alkyl groups on the amide nitrogen were probed together with the effects of halogen substituents on one or both of the aryl rings. Some ligands were also synthesized for study which represent conformationally constrained versions of the parent structure. Broad screening studies revealed these indoleacetamides to be highly selective for the mDRC, since they failed to bind with any significant affinity to other receptor systems. Some of the ligands were found to exhibit Ki values in the low nanomolar range for the mDRC as measured by the displacement of [3H]4'-chlorodiazepam. A subset of these ligands was also shown to stimulate pregnenolone formation from the mitochondria of C6-2B glioma cells with an EC50 of about 3 nM. In animal experiments ligands selected for further study were found to exhibit antineophobic effects, in spite of the fact that they exhibit no direct action on GABAA receptors. Consequently, it is postulated that these ligands owe their action to an indirect modulation of GABAA receptor function, presumably by stimulation of neurosteroid production and release from glial cells, followed by neurosteroid modulation of GABA's action on the chloride ion channel conductance of GABAA receptors.


