EvolitrineCAS# 523-66-0 |
Quality Control & MSDS
Number of papers citing our products

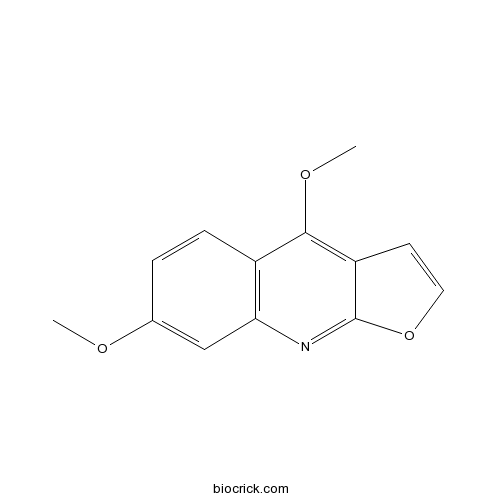
Chemical structure

3D structure
| Cas No. | 523-66-0 | SDF | Download SDF |
| PubChem ID | 196980 | Appearance | Yellow crystal |
| Formula | C13H11NO3 | M.Wt | 229.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4,7-dimethoxyfuro[2,3-b]quinoline | ||
| SMILES | COC1=CC2=C(C=C1)C(=C3C=COC3=N2)OC | ||
| Standard InChIKey | TWGHMXOYRUTQOL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H11NO3/c1-15-8-3-4-9-11(7-8)14-13-10(5-6-17-13)12(9)16-2/h3-7H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Evolitrine Dilution Calculator

Evolitrine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3624 mL | 21.8122 mL | 43.6243 mL | 87.2486 mL | 109.0608 mL |
| 5 mM | 0.8725 mL | 4.3624 mL | 8.7249 mL | 17.4497 mL | 21.8122 mL |
| 10 mM | 0.4362 mL | 2.1812 mL | 4.3624 mL | 8.7249 mL | 10.9061 mL |
| 50 mM | 0.0872 mL | 0.4362 mL | 0.8725 mL | 1.745 mL | 2.1812 mL |
| 100 mM | 0.0436 mL | 0.2181 mL | 0.4362 mL | 0.8725 mL | 1.0906 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Angelicin
Catalog No.:BCN5669
CAS No.:523-50-2
- 4-Amino-2,5-dimethoxy-N-phenylbenzenesulphonamide
Catalog No.:BCC8676
CAS No.:52298-44-9
- Ginsenoside Rg2
Catalog No.:BCN1067
CAS No.:52286-74-5
- Ginsenoside Re
Catalog No.:BCN1073
CAS No.:52286-59-6
- Ginsenoside Rf
Catalog No.:BCN1075
CAS No.:52286-58-5
- 3,5-Diprenyl-4-hydroxybenzaldehyde
Catalog No.:BCN4624
CAS No.:52275-04-4
- CGP 57380
Catalog No.:BCC5279
CAS No.:522629-08-9
- Isomucronulatol
Catalog No.:BCN1428
CAS No.:52250-35-8
- Parathyroid hormone (1-34) (human)
Catalog No.:BCC1046
CAS No.:52232-67-4
- Kaempferol-4'-O-beta-D-glucopyranoside
Catalog No.:BCN8130
CAS No.:52222-74-9
- Ciprofibrate
Catalog No.:BCC2266
CAS No.:52214-84-3
- 3-Epicorosolic acid
Catalog No.:BCN5666
CAS No.:52213-27-1
- Flavoglaucin
Catalog No.:BCN6398
CAS No.:523-73-9
- Anisatin
Catalog No.:BCC8118
CAS No.:5230-87-5
- Vindorosine
Catalog No.:BCN5668
CAS No.:5231-60-7
- p,p-hydroxy-curucumin
Catalog No.:BCC8890
CAS No.:52328-96-8
- Tetramethylcurcumin
Catalog No.:BCN2746
CAS No.:52328-97-9
- Dimethylcurcumin
Catalog No.:BCN2748
CAS No.:52328-98-0
- 6alpha-Chloro-5beta-hydroxywithaferin A
Catalog No.:BCN8007
CAS No.:52329-20-1
- Alnusone
Catalog No.:BCN8108
CAS No.:52330-11-7
- 2-Benzoyl-1,3,4,4a,5,8a-hexahydro-6(2H)-isoquinolinone
Catalog No.:BCC8559
CAS No.:52346-14-2
- Erysotramidine
Catalog No.:BCN5670
CAS No.:52358-58-4
- Epistephamiersine
Catalog No.:BCN5671
CAS No.:52389-15-8
- Wedelolactone
Catalog No.:BCN5672
CAS No.:524-12-9
Quinoline alkaloids from Acronychia laurifolia.[Pubmed:10466225]
Phytochemistry. 1999 Sep;52(1):95-8.
Bioassay-directed fractionation of a root extract of Acronychia laurifolia (Rutaceae) using the KB-V1+ human tumor cell line led to the isolation of six quinoline alkaloids. One of these alkaloids is novel, namely, 2,3-methylenedioxy-4,7-dimethoxyquinoline and the other five were identified as the known compounds, Evolitrine, gamma-fagarine, skimmianine, kokusaginine and maculosidine. Two known bis-tetrahydrofuran lignans, sesamolin and yangambin, were also identified. The structure of the new alkaloid was determined by spectroscopic methods. All of the isolates were evaluated against a panel of human cancer cell lines; four of the alkaloids showed weak cytotoxic activity.
Alkaloids from Ruta montana.[Pubmed:10680183]
Phytochemistry. 2000 Jan;53(2):277-9.
Two known and four new quinoline and 4-quinolone type alkaloids were isolated from Ruta montana collected from Rommani (Morocco). The known compounds were 1-methyl-4-methoxy-2-quinolone and Evolitrine. The structures of the new compounds were established from 1D and 2D NMR experiments including HMQC, HMBC and MS spectral methods as 2-(nonan-8-one)-(1H)-4-quinolone, 2-(nonan-8-one)-4-methoxy-quinoline, 2-(nonan-8-one)-N-methyl-4-quinolone and 2-(decan-9-one)-N-methyl-4-quinolone.
Furoquinoline alkaloids as photosensitizers in Chlamydomonas reinhardtii.[Pubmed:2067526]
Mutat Res. 1991 Jul;249(1):105-10.
Seven naturally occurring furoquinoline alkaloids were investigated for their photobiological activity using arg-1 cells of Chlamydomonas reinhardtii. UV-A-mediated toxicity of the compounds was calculated from the colony-forming ability of the treated cells. The UV-A-mediated mutagenicity was measured by counting the number of Arg+ revertants induced by the treatment. Dictamnine was found to be the strongest mutagen as well as the most toxic compound of the group. The mutagenic activities were measured as mutation frequencies at equal substance concentration and ranked in the following order: An increase in the number of substituents on the lateral aromatic nucleus greatly decreased the photomutagenicity. Except for Evolitrine, a similar ranking order was found as reported for the dark mutagenicity of these compounds in Salmonella typhimurium strain TA98. Based on the result that furoquinolines are able to intercalate into DNA, we assume that the different mutagenic potencies may reflect differences in the geometry of the intercalation complex, which is important for the subsequent photochemical reaction.
Furoquinoline alkaloids isolated from Balfourodendron riedelianum as photosynthetic inhibitors in spinach chloroplasts.[Pubmed:23416711]
J Photochem Photobiol B. 2013 Mar 5;120:36-43.
In the search for natural inhibitors of plant growth, we investigate the mechanism of action of the natural furoquinoline alkaloids isolated from Balfourodendron riedelianum (Rutaceae): Evolitrine (1), kokusaginine (2), gamma-fagarine (3), skimmianine (4) and maculosidine (5) on the photosynthesis light reactions. Their effect on the electron transport chain on thylakoids was analyzed. Alkaloids 1, 2, 4 and 5 inhibited ATP synthesis, basal, phosphorylating and uncoupled electron transport acting as Hill reaction inhibitors on spinach chloroplasts. Alkaloid 3 was not active. The inhibition and interaction site of alkaloids 1, 2, 4 and 5 on the non-cyclic electron transport chain was studied by polarography and fluorescence of the chlorophyll a (Chl a). The results indicate that the target for 1 was localized on the donor and acceptor side of PS II. In addition alkaloids 2 and 5 affect the PS I electron acceptors on leaf discs.
Inhibitory effects of furoquinoline alkaloids from Melicope confusa and Dictamnus albus against human phosphodiesterase 5 (hPDE5A) in vitro.[Pubmed:16042076]
Arch Pharm Res. 2005 Jun;28(6):675-9.
Eight furoquinoline alkaloids were purified from two plants belonging to the Rutaceae family. Kokusaginine, skimmianine, Evolitrine, and confusameline were purified from Melicope confusa, and haplopine, robustine, dictamine, and gamma-fagarine from Dictamnus albus. In this study, the eight furoquinoline alkaloids were examined for inhibitory potency against human phosphodiesterase 5 (hPDE5A) in vitro. DNA encoding the catalytic domain of human PDE5A was amplified from the mRNA of T24 cells by RT-PCR and was fused to GST in an expression vector. GST-tagged PDE5A was then purified by glutathione affinity chromatography and used in inhibition assays. Of the eight alkaloids, gamma-fagarine was the most potent inhibitor of PDE5A, and its single methoxy group at the C-8 position was shown to be critical for inhibitory activity. These results clearly illustrate the relationship between PDE5A inhibition and the methoxy group position in furoquinoline alkaloids.
A new quinoline epoxide from the Australian plant Drummondita calida.[Pubmed:21472648]
Planta Med. 2011 Sep;77(14):1644-7.
A drug discovery program aimed at identifying new antimalarial leads from a prefractionated natural product library has resulted in the purification of a new quinoline alkaloid, (2' R)-2',3'-epoxy- N-methylatanine (1), along with eight known natural products, skimmianin, gamma-fagarine, maculosidine, Evolitrine, dictamnine, pteleine, N-methylatanine, and werneria chromene. Compound 1 displayed 74 % inhibition at 80 microM against a chloroquine -resistant Plasmodium falciparum strain (Dd2).


