EupatoriopicrinCAS# 6856-01-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6856-01-5 | SDF | Download SDF |
| PubChem ID | 5281461 | Appearance | Powder |
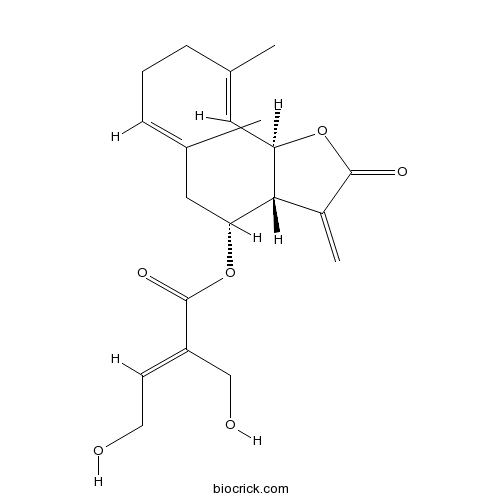
| Formula | C20H26O6 | M.Wt | 362.42 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(3aR,4R,6E,10E,11aR)-6,10-dimethyl-3-methylidene-2-oxo-3a,4,5,8,9,11a-hexahydrocyclodeca[b]furan-4-yl] (E)-4-hydroxy-2-(hydroxymethyl)but-2-enoate | ||
| SMILES | CC1=CC2C(C(CC(=CCC1)C)OC(=O)C(=CCO)CO)C(=C)C(=O)O2 | ||
| Standard InChIKey | VWJYWGYJIDQUEG-DKDOXNMLSA-N | ||
| Standard InChI | InChI=1S/C20H26O6/c1-12-5-4-6-13(2)10-17(26-20(24)15(11-22)7-8-21)18-14(3)19(23)25-16(18)9-12/h6-7,9,16-18,21-22H,3-5,8,10-11H2,1-2H3/b12-9+,13-6+,15-7+/t16-,17-,18+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Eupatoriopicrin shows anti-trypanosomal activities and cytotoxicity against Trypanosoma brucei rhodesiense. 2. Eupatoriopicrin shows a weak sensitizing capacity in guinea pigs. 3. Eupatoriopicrin-induced DNA damage may play a role in the observed cytotoxicity. |
| Targets | Antifection |

Eupatoriopicrin Dilution Calculator

Eupatoriopicrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7592 mL | 13.7961 mL | 27.5923 mL | 55.1846 mL | 68.9807 mL |
| 5 mM | 0.5518 mL | 2.7592 mL | 5.5185 mL | 11.0369 mL | 13.7961 mL |
| 10 mM | 0.2759 mL | 1.3796 mL | 2.7592 mL | 5.5185 mL | 6.8981 mL |
| 50 mM | 0.0552 mL | 0.2759 mL | 0.5518 mL | 1.1037 mL | 1.3796 mL |
| 100 mM | 0.0276 mL | 0.138 mL | 0.2759 mL | 0.5518 mL | 0.6898 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GSK 264220A
Catalog No.:BCC6062
CAS No.:685506-42-7
- Cilostamide
Catalog No.:BCC6843
CAS No.:68550-75-4
- Isoguvacine hydrochloride
Catalog No.:BCC6575
CAS No.:68547-97-7
- Angiotensin II
Catalog No.:BCC1030
CAS No.:68521-88-0
- Vigabatrin
Catalog No.:BCC2039
CAS No.:68506-86-5
- Pramiracetam
Catalog No.:BCC4928
CAS No.:68497-62-1
- WS 12
Catalog No.:BCC7543
CAS No.:68489-09-8
- Coromandaline
Catalog No.:BCN2044
CAS No.:68473-86-9
- Heliovicine
Catalog No.:BCN2047
CAS No.:68473-85-8
- 4-(p-Biphenylyl)-3-hydroxybutyric acid
Catalog No.:BCN2240
CAS No.:6845-17-6
- Isowighteone
Catalog No.:BCN4243
CAS No.:68436-47-5
- PPDA
Catalog No.:BCC5918
CAS No.:684283-16-7
- Pridinol Methanesulfonate
Catalog No.:BCC3845
CAS No.:6856-31-1
- Moschamine
Catalog No.:BCN3900
CAS No.:68573-23-9
- Prometaphanine
Catalog No.:BCN4244
CAS No.:6858-85-1
- PX-478 2HCl
Catalog No.:BCC6502
CAS No.:685898-44-6
- Isorhyncophylline
Catalog No.:BCN3466
CAS No.:6859-01-4
- Isorhynchophylline
Catalog No.:BCN6458
CAS No.:6859-1-4
- Xylobiose
Catalog No.:BCN8424
CAS No.:6860-47-5
- Procerine
Catalog No.:BCN2017
CAS No.:68622-81-1
- Otenabant
Catalog No.:BCC1828
CAS No.:686344-29-6
- CP-945598 HCl
Catalog No.:BCC1082
CAS No.:686347-12-6
- BOP-Cl
Catalog No.:BCC2808
CAS No.:68641-49-6
- (±)-Palmitoylcarnitine chloride
Catalog No.:BCC6718
CAS No.:6865-14-1
Antitrypanosomal sesquiterpene lactones from Saussurea costus.[Pubmed:21624443]
Fitoterapia. 2011 Oct;82(7):955-9.
In the course of a larger screen of 1800 plant and fungal extracts, the ethyl acetate extract of Saussurea costus roots potently inhibited the growth of Trypanosoma brucei rhodesiense. Subsequent HPLC based activity profiling led to the identification of the sesquiterpene lactones arbusculin B (1), alpha-cyclocostunolide (2), costunolide (3), and dehydrocostuslactone (4). They were tested for in vitro antitrypanosomal activities and cytotoxicity alongside the structurally related sesquiterpene lactones parthenolide (5), zaluzanin D (6), and Eupatoriopicrin (7), and had IC(50)s between 0.8 and 22 muM. Cytotoxic IC(50)s were from 1.6 to 19 muM, and selectivity indices from 0.5 to 6.5.
Allergenic sesquiterpene lactones from Eupatorium cannabinum L. and Kaunia rufescens (Lund ex de Candolle).[Pubmed:9615309]
Nat Toxins. 1997;5(6):223-7.
From Eupatorium cannabinum L., a hitherto unknown alpha-methylene-gamma-butyrolactone, 3 beta-peroxyeucannabinolide, was isolated. This compound and Eupatoriopicrin from the same plant showed a weak sensitizing capacity in guinea pigs. 2-oxoludartin and dehydroleucodin, isolated from Kaunia rufescens (syn. Eupatorium rufescens), were strong sensitizers in the same sensitizarian procedure.
Induction of DNA damage in Ehrlich ascites tumour cells by exposure to eupatoriopicrin.[Pubmed:2751694]
Biochem Pharmacol. 1989 Jul 15;38(14):2279-83.
The sesquiterpene lactone Eupatoriopicrin (EUP) from Eupatorium cannabinum L. has been shown to be cytotoxic in a glutathione (GSH)-dependent way. In order to assess possible DNA damage as a cause for cell death, the study reported was initiated. After 2 hr incubation of Ehrlich ascites tumour cells with EUP, the DNA damage, determined by the use of an alkaline DNA unwinding method, followed by hydroxylapatite column chromatography of degraded DNA, was observed at concentrations only slightly higher than those causing cell death in a clonogenic assay. The amount of EUP, requested to demonstrate DNA damage after a 24-hr post-incubation period lay within the concentration range that was effective in the clonogenic assay (1-10 micrograms/ml). Glutathione (GSH) depletion of the cells to about 99%, by use of buthionine sulphoximine (BSO), enhanced the extent of DNA damage. It is concluded that EUP-induced DNA damage may play a role in the observed cytotoxicity.


