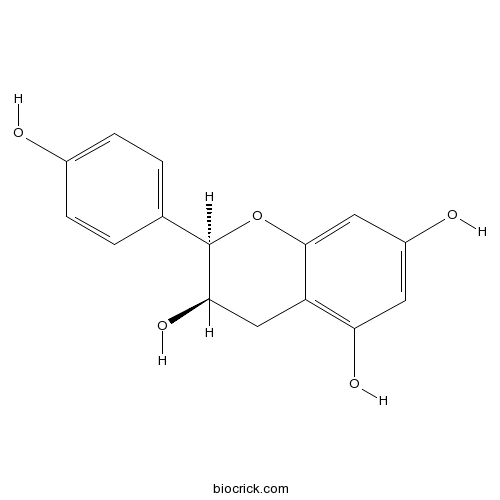
(-)-EpiafzelechinCAS# 24808-04-6 |
- (+)-Afzelechin
Catalog No.:BCN5121
CAS No.:2545-00-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 24808-04-6 | SDF | Download SDF |
| PubChem ID | 443639 | Appearance | Powder |
| Formula | C15H14O5 | M.Wt | 274.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R)-2-(4-hydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | ||
| SMILES | C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC=C(C=C3)O)O | ||
| Standard InChIKey | RSYUFYQTACJFML-UKRRQHHQSA-N | ||
| Standard InChI | InChI=1S/C15H14O5/c16-9-3-1-8(2-4-9)15-13(19)7-11-12(18)5-10(17)6-14(11)20-15/h1-6,13,15-19H,7H2/t13-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (-)-Epiafzelechin exhibits a dose-dependent inhibition on the COX activity with an IC50 value of 15 microM, it exhibits about 3-fold weaker inhibitory potency on the enzyme activity than indomethacin as a positive control. 2. (-)-Epiafzelechin exhibits significant anti-inflammatory activity on carrageenin-induced mouse paw edema when the compound (100 mg/kg) was orally administrated at 1 h before carrageenin treatment. 3. (-)-Epicatechin shows zero and/or the lowest activities against pancreatic lipase (IC50 > 20 microM). 4. Epiafzelechin has antioxidant properties. |
| Targets | COX |

(-)-Epiafzelechin Dilution Calculator

(-)-Epiafzelechin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6456 mL | 18.2282 mL | 36.4564 mL | 72.9129 mL | 91.1411 mL |
| 5 mM | 0.7291 mL | 3.6456 mL | 7.2913 mL | 14.5826 mL | 18.2282 mL |
| 10 mM | 0.3646 mL | 1.8228 mL | 3.6456 mL | 7.2913 mL | 9.1141 mL |
| 50 mM | 0.0729 mL | 0.3646 mL | 0.7291 mL | 1.4583 mL | 1.8228 mL |
| 100 mM | 0.0365 mL | 0.1823 mL | 0.3646 mL | 0.7291 mL | 0.9114 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-Orn(Z)-OH
Catalog No.:BCC3430
CAS No.:2480-93-5
- N2-Methyl-L-arginine
Catalog No.:BCC6032
CAS No.:2480-28-6
- H-N-Me-Ser-OH.HCl
Catalog No.:BCC3352
CAS No.:2480-26-4
- N-Me-Val-OH.HCl
Catalog No.:BCC2612
CAS No.:2480-23-1
- Estriol 3-glucuronide
Catalog No.:BCN2239
CAS No.:2479-91-6
- 1,3-Bis(4-aminophenoxy)benzene
Catalog No.:BCC8418
CAS No.:2479-46-1
- Beta-Peltoboykinolic acid
Catalog No.:BCN6635
CAS No.:24778-48-1
- SB 328437
Catalog No.:BCC6056
CAS No.:247580-43-4
- Leonurin monohydrochloride
Catalog No.:BCN8304
CAS No.:24735-18-0
- Dihydroergocristine mesylate
Catalog No.:BCC6657
CAS No.:24730-10-7
- Fimasartan
Catalog No.:BCC5552
CAS No.:247257-48-3
- Daphnezomine B
Catalog No.:BCN5113
CAS No.:247078-43-9
- H-Cys(tBu)-OH.HCl
Catalog No.:BCC2910
CAS No.:2481-09-6
- Reserpinine
Catalog No.:BCN3490
CAS No.:24815-24-5
- Agmatine sulfate
Catalog No.:BCC6813
CAS No.:2482-00-0
- Laquinimod (ABR-215062)
Catalog No.:BCC3802
CAS No.:248281-84-7
- Boc-Lys(Boc)-OH
Catalog No.:BCC3412
CAS No.:2483-46-7
- Azaphen
Catalog No.:BCC1390
CAS No.:24853-80-3
- Erlotinib mesylate
Catalog No.:BCC1558
CAS No.:248594-19-6
- Boc-Met-OH
Catalog No.:BCC3424
CAS No.:2488-15-5
- Mesembrine
Catalog No.:BCN3668
CAS No.:24880-43-1
- Bakkenolide III
Catalog No.:BCN7245
CAS No.:24909-95-3
- H-Met-OMe. HCl
Catalog No.:BCC2995
CAS No.:2491-18-1
- H-Ala-OMe.HCl
Catalog No.:BCC3192
CAS No.:2491-20-5
Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro.[Pubmed:15913331]
J Agric Food Chem. 2005 Jun 1;53(11):4593-8.
Fifty-four polyphenols isolated from tea leaves were evaluated for their inhibitory activities against pancreatic lipase, the key enzyme of lipid absorption in the gut. (-)-Epigallocatechin 3-O-gallate (EGCG), which is one of major polyphenols in green tea, showed lipase inhibition with an IC50 of 0.349 microM. Moreover, flavan-3-ol digallate esters, such as (-)-epigallocatechin-3,5-digallate, showed higher activities of inhibition on lipase with an IC50 of 0.098 microM. On the other hand, nonesterified flavan-3-ols, such as (+)-catechin, (-)-epicatechin, (+)-gallocatechin, and (-)-epigallocatechin, showed zero and/or the lowest activities against pancreatic lipase (IC50 > 20 microM). These data suggested that the presence of galloyl moieties within the structure was required for enhancement of pancreatic lipase inhibition. It is well-known that flavan-3-ols are polymerized by polyphenol oxidase and/or heating in a manufacturing process of oolong tea. Oolonghomobisflavans A and B and oolongtheanin 3'-O-gallate, which are typical in oolong tea leaves, showed strong inhibitory activities with IC50 values of 0.048, 0.108, and 0.068 microM, respectively, even higher than that of EGCG. The oolong tea polymerized polyphenols (OTPP) were prepared for the assay from oolong tea extract, from which the preparation effectively subtracted the zero and/or less-active monomeric flavan-3-ols by preparative high-performance liquid chromatography. The weight-average molecular weight (Mw) and number-average molecular-weight (Mn) values of OTPP were 2017 and 903, respectively, by using gel permeation choromatography. OTPP showed a 5-fold stronger inhibition against pancreatic lipase (IC50 = 0.28 microg/mL) by comparison with that of the tannase-treated OTPP (IC50 = 1.38 microg/mL). These data suggested that the presence of galloyl moieties within their chemical structures and/or the polymerization of flavan-3-ols were required for enhancement of pancreatic lipase inhibition.
(-)-Epiafzelechin: cyclooxygenase-1 inhibitor and anti-inflammatory agent from aerial parts of Celastrus orbiculatus.[Pubmed:10418338]
Planta Med. 1999 Jun;65(5):460-2.
An inhibitor of cyclooxygenase (COX)-1 activity of prostaglandin H2 synthase was isolated from aerial parts of Celastrus orbiculatus Thunb. (Celastraceae), an oriental folk medicine for rheumatoid arthritis by activity-guided column chromatographic methods. The COX inhibitor was identified as (-)-Epiafzelechin, a member of flavan-3-ols by the structural analysis with HR-EI-mass, 1H-NMR and 13C-NMR spectral data. The compound exhibited a dose-dependent inhibition on the COX activity with an IC50 value of 15 microM. (-)-Epiafzelechin exhibited about 3-fold weaker inhibitory potency on the enzyme activity than indomethacin as a positive control. (-)-Epiafzelechin exhibited significant anti-inflammatory activity on carrageenin-induced mouse paw edema when the compound (100 mg/kg) was orally administrated at 1 h before carrageenin treatment.
Epiafzelechin from the root bark of Cassia sieberiana: detection by DART mass spectrometry, spectroscopic characterization, and antioxidant properties.[Pubmed:21070009]
J Nat Prod. 2011 Mar 25;74(3):455-9.
The root bark of Cassia sieberiana was analyzed using direct analysis in real time mass spectrometry, and a main flavonoid component with an [M + H](+) mass of 275 was identified. The flavonoid, epiafzelechin, was isolated and fully characterized with the concerted use of NMR spectroscopy, circular dichroism, and optical rotation. Electronic circular dichroism and optical rotation TDDFT calculations were also performed, and their agreement with the experimental results confirmed the enantiomeric identity of the isolated natural product. The antioxidant activity of the compound was also investigated.


