Emodin-8-beta-D-glucosideCAS# 23313-21-5 |
Quality Control & MSDS
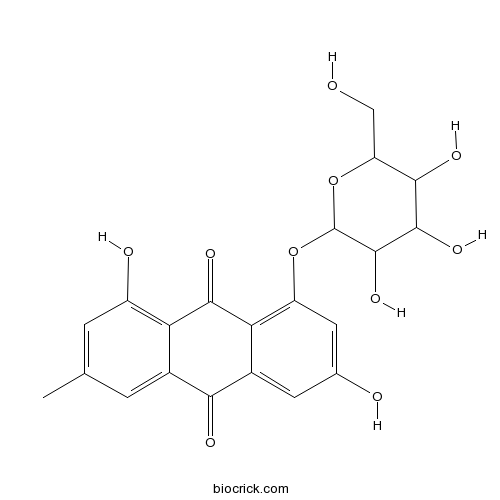
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23313-21-5 | SDF | Download SDF |
| PubChem ID | 318730 | Appearance | Orange powder |
| Formula | C21H20O10 | M.Wt | 432.41 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Synonyms | Anthraglycoside B; 1,3,8-Trihydroxy 6-methyl 9,10-anthraquinone 8-glucoside | ||
| Solubility | Soluble in methanol and pyridine | ||
| Chemical Name | 1,6-dihydroxy-3-methyl-8-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyanthracene-9,10-dione | ||
| SMILES | CC1=CC(=C2C(=C1)C(=O)C3=CC(=CC(=C3C2=O)OC4C(C(C(C(O4)CO)O)O)O)O)O | ||
| Standard InChIKey | HSWIRQIYASIOBE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H20O10/c1-7-2-9-14(11(24)3-7)18(27)15-10(16(9)25)4-8(23)5-12(15)30-21-20(29)19(28)17(26)13(6-22)31-21/h2-5,13,17,19-24,26,28-29H,6H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Emodin-8-beta-D-glucoside functions to protect from focal cerebral injury induced by ischemia and reperfusion. It directly stimulates cell proliferation and differentiation of osteoblasts. |
| Targets | PGE | TNF-α |
| In vitro | Emodin-8-O-β-D-glucoside from Polygonum amplexicaule D. Don var. sinense Forb. promotes proliferation and differentiation of osteoblastic MC3T3-E1 cells.[Pubmed: 21245807 ]Molecules. 2011 Jan 18;16(1):728-37.Polygonum amplexicaule D. Don var. sinense Forb. (Polygonaceae) (PAF) is a famous traditional herb used to treat fractures, rheumatoid arthritis, muscle injury and pain. |
| In vivo | Neuroprotective effects of emodin-8-O-beta-D-glucoside in vivo and in vitro.[Pubmed: 17897641 ]Eur J Pharmacol. 2007 Dec 22;577(1-3):58-63.Emodin-8-O-beta-D-glucoside(Emodin-8-beta-D-glucoside) extracted from the traditional Chinese medicinal herb Polygonum cuspidatum Sieb. et Zucc is widely used to treat acute hepatitis possibly by antioxidative mechanisms. The present study was designed to investigate whether emodin-8-O-beta-D-glucoside(Emodin-8-beta-D-glucoside)exerted neuroprotective effects on the focal cerebral injury induced by ischemia and reperfusion in vivo and on the neuronal damage induced by glutamate in vitro, and to study the possible mechanisms. |
| Structure Identification | J Chromatogr A. 2006 May 19;1115(1-2):64-71.Preparative isolation and purification of chemical constituents from the root of Polygonum multiflorum by high-speed counter-current chromatography.[Pubmed: 16564530]High-speed counter-current chromatography methods, combined with solvent partition, were applied to the systematic separation and purification of chemical components from Chinese medicinal herb Polygonum multiflorum extract. The aim of this paper is summing up the rules of solvent system selection for diverse fractions of herbal extract, and establishing the systematic pattern to screen the bioactive constituents rapidly. |

Emodin-8-beta-D-glucoside Dilution Calculator

Emodin-8-beta-D-glucoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3126 mL | 11.5631 mL | 23.1262 mL | 46.2524 mL | 57.8155 mL |
| 5 mM | 0.4625 mL | 2.3126 mL | 4.6252 mL | 9.2505 mL | 11.5631 mL |
| 10 mM | 0.2313 mL | 1.1563 mL | 2.3126 mL | 4.6252 mL | 5.7815 mL |
| 50 mM | 0.0463 mL | 0.2313 mL | 0.4625 mL | 0.925 mL | 1.1563 mL |
| 100 mM | 0.0231 mL | 0.1156 mL | 0.2313 mL | 0.4625 mL | 0.5782 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Delta 7-avenasterol
Catalog No.:BCN3212
CAS No.:23290-26-8
- Probucol
Catalog No.:BCC4833
CAS No.:23288-49-5
- m-NH2-Tyr-OH.2HCl
Catalog No.:BCC3340
CAS No.:23279-22-3
- VAL-083
Catalog No.:BCC2024
CAS No.:23261-20-3
- Bay 36-7620
Catalog No.:BCC5915
CAS No.:232605-26-4
- 4'-O-Methylvitexin
Catalog No.:BCN2642
CAS No.:2326-34-3
- Guanabenz Acetate
Catalog No.:BCC4327
CAS No.:23256-50-0
- Dimaprit dihydrochloride
Catalog No.:BCC6672
CAS No.:23256-33-9
- Riddelline
Catalog No.:BCN2133
CAS No.:23246-96-0
- 5,7-Diacetoxy-8-methoxyflavone
Catalog No.:BCN5083
CAS No.:23246-80-2
- Ritodrine HCl
Catalog No.:BCC4337
CAS No.:23239-51-2
- TR-14035
Catalog No.:BCC4266
CAS No.:232271-19-1
- Cephalexin monohydrate
Catalog No.:BCC4096
CAS No.:23325-78-2
- Nefopam HCl
Catalog No.:BCC4681
CAS No.:23327-57-3
- MK 0343
Catalog No.:BCC6170
CAS No.:233275-76-8
- Glycoborinine
Catalog No.:BCN7462
CAS No.:233279-39-5
- L-Ser(Bzl)-ol
Catalog No.:BCC2579
CAS No.:23356-96-9
- Vinleurosine
Catalog No.:BCN2608
CAS No.:23360-92-1
- (1S,2R)-2-Amino-1,2-diphenylethanol
Catalog No.:BCC8385
CAS No.:23364-44-5
- Theviridoside
Catalog No.:BCN5084
CAS No.:23407-76-3
- Phalaenopsine T
Catalog No.:BCN2014
CAS No.:23412-97-7
- Phalaenopsine La
Catalog No.:BCN2015
CAS No.:23412-99-9
- 7-Isopentenyloxy-gamma-fagarine
Catalog No.:BCN5085
CAS No.:23417-92-7
- Tetrahydropiperin
Catalog No.:BCN6708
CAS No.:23434-88-0
Emodin-8-O-beta-D-glucoside from Polygonum amplexicaule D. Don var. sinense Forb. promotes proliferation and differentiation of osteoblastic MC3T3-E1 cells.[Pubmed:21245807]
Molecules. 2011 Jan 18;16(1):728-37.
Polygonum amplexicaule D. Don var. sinense Forb. (Polygonaceae) (PAF) is a famous traditional herb used to treat fractures, rheumatoid arthritis, muscle injury and pain. The present study was designed to investigate a PAF derived-chemical compound emodin-8-O-beta-D-glucoside (EG) on the proliferation and differentiation of osteoblastic MC3T3-E1 cell in vitro. A compound was isolated from PAF extract by HPLC and identified as emodin-8-O-beta-D-glucoside (EG) by spectroscopic methods. EG significantly promoted cell proliferation at 0.1-100 ng/mL, and increased the cell proportion in S-phase from 16.34% to 32.16%. Moreover, EG increased alkaline phosphatase (ALP) expression in MC3T3-E1 cells at the concentration from 0.1 to 100 ng/mL and inhibited PGE(2 )production induced by TNF-alpha in osteoblasts at the concentrations ranging from 10-100 ng/mL, suggesting that cell differentiation was induced in MC3T3-E1 osteoblasts. Taken together, these results indicated compound EG directly stimulated cell proliferation and differentiation of osteoblasts, therefore this study preliminarily explored the pharmacological mechanism of PAF to promote the healing of bone rheumatism and various fractures.
Neuroprotective effects of emodin-8-O-beta-D-glucoside in vivo and in vitro.[Pubmed:17897641]
Eur J Pharmacol. 2007 Dec 22;577(1-3):58-63.
Emodin-8-O-beta-D-glucoside extracted from the traditional Chinese medicinal herb Polygonum cuspidatum Sieb. et Zucc is widely used to treat acute hepatitis possibly by antioxidative mechanisms. The present study was designed to investigate whether emodin-8-O-beta-D-glucoside exerted neuroprotective effects on the focal cerebral injury induced by ischemia and reperfusion in vivo and on the neuronal damage induced by glutamate in vitro, and to study the possible mechanisms. Male Wistar rats were used to establish the model of ischemia and reperfusion. The behavioral test was performed and the cerebral infarction area was assessed in the brain slices stained with 2% 2,3,5-triphenyl tetrazolium chloride to evaluate the neuroprotective effects of emodin-8-O-beta-D-glucoside. Superoxide dismutase (SOD) activity, total antioxidative capability and malondialdehyde (MDA) level in the brain tissue were determined with spectrophotometrical methods to probe the primary mechanisms of emodin-8-O-beta-D-glucoside. In vitro, the neuroprotective effects of emodin-8-O-beta-D-glucoside were tested in the cultured cortical cells of fetal rats exposed to glutamate. Emodin-8-O-beta-D-glucoside concentration in plasma and brain tissue was also measured to examine distribution of emodin-8-O-beta-D-glucoside in the brain. The results showed that the treatment of rats with emodin-8-O-beta-D-glucoside reduced the neurological deficit score and the cerebral infarction area, increased SOD activity and total antioxidative capability, and decreased MDA level in the brain tissue in dose-dependent way. Emodin-8-O-beta-D-glucoside also inhibited the neuronal damage induced by glutamate. Besides, emodin-8-O-beta-D-glucoside was able to penetrate blood-brain barrier and distribute in the brain tissue. These findings demonstrate that emodin-8-O-beta-D-glucoside is able to provide neuroprotection against cerebral ischemia-reperfused injury and glutamate induced neuronal damage through exerting antioxidative effects and inhibiting glutamate neurotoxicity.
Preparative isolation and purification of chemical constituents from the root of Polygonum multiflorum by high-speed counter-current chromatography.[Pubmed:16564530]
J Chromatogr A. 2006 May 19;1115(1-2):64-71.
High-speed counter-current chromatography methods, combined with solvent partition, were applied to the systematic separation and purification of chemical components from Chinese medicinal herb Polygonum multiflorum extract. The aim of this paper is summing up the rules of solvent system selection for diverse fractions of herbal extract, and establishing the systematic pattern to screen the bioactive constituents rapidly. Nine compounds including emodin, chrysophanol, rhein, 6-OH-emodin, Emodin-8-beta-D-glucoside, polygonimitin B, 2,3,5,4'-tetrahydroxystilbene-2-beta-D-glucoside, gallic acid and an unknown glycoside, which differed in quantity and polarity remarkably, were obtained. The purities of them were all above 97% as determined by high-performance liquid chromatography (HPLC), and their structures were identified by 1H NMR and electrospray ionization mass spectrometry (ESI-MS). The results demonstrated that HSCCC is a speedy and efficient technique for systematic isolation of bioactive components from traditional medicinal herbs.


