EctoineCAS# 96702-03-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 96702-03-3 | SDF | Download SDF |
| PubChem ID | 126041 | Appearance | Powder |
| Formula | C6H10N2O2 | M.Wt | 142.16 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
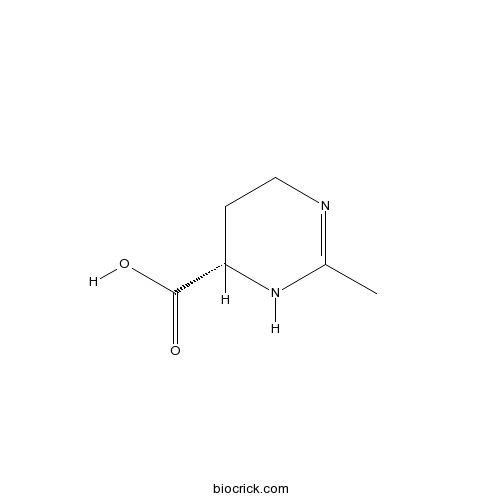
| Chemical Name | (6S)-2-methyl-1,4,5,6-tetrahydropyrimidine-6-carboxylic acid | ||
| SMILES | CC1=NCCC(N1)C(=O)O | ||
| Standard InChIKey | WQXNXVUDBPYKBA-YFKPBYRVSA-N | ||
| Standard InChI | InChI=1S/C6H10N2O2/c1-4-7-3-2-5(8-4)6(9)10/h5H,2-3H2,1H3,(H,7,8)(H,9,10)/t5-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ectoine Dilution Calculator

Ectoine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.0343 mL | 35.1716 mL | 70.3433 mL | 140.6866 mL | 175.8582 mL |
| 5 mM | 1.4069 mL | 7.0343 mL | 14.0687 mL | 28.1373 mL | 35.1716 mL |
| 10 mM | 0.7034 mL | 3.5172 mL | 7.0343 mL | 14.0687 mL | 17.5858 mL |
| 50 mM | 0.1407 mL | 0.7034 mL | 1.4069 mL | 2.8137 mL | 3.5172 mL |
| 100 mM | 0.0703 mL | 0.3517 mL | 0.7034 mL | 1.4069 mL | 1.7586 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Secoisolariciresinoldiglucoside
Catalog No.:BCN9183
CAS No.:257930-74-8
- Bisisorhapontigenin G
Catalog No.:BCN9182
CAS No.:
- 2α-Acetoxy-14,15-cyclopimara-7β,16-diol
Catalog No.:BCN9181
CAS No.:2011034-27-6
- 4′-O-Isobutyroylpeguangxienin
Catalog No.:BCN9180
CAS No.:2188162-95-8
- (±)-Pinoresinol
Catalog No.:BCN9179
CAS No.:4263-88-1
- Dehydroevodiamine hydrochloride
Catalog No.:BCN9178
CAS No.:111664-82-5
- Tinosporol C
Catalog No.:BCN9177
CAS No.:2244777-14-6
- Tinosporoside A
Catalog No.:BCN9176
CAS No.:2244777-15-7
- Tinosporol A
Catalog No.:BCN9175
CAS No.:2244777-12-4
- Tinosporol B
Catalog No.:BCN9174
CAS No.:2244777-13-5
- Epimedonin H
Catalog No.:BCN9173
CAS No.:2222285-82-5
- Epimedonin G
Catalog No.:BCN9172
CAS No.:2222285-80-3
- Stachartone A
Catalog No.:BCN9185
CAS No.:2209109-64-6
- Aspergillon A
Catalog No.:BCN9186
CAS No.:2239299-08-0
- Bistachybotrysin E
Catalog No.:BCN9187
CAS No.:2231761-98-9
- Ustusol H4
Catalog No.:BCN9188
CAS No.:2193060-25-0
- Shiraiachrome A
Catalog No.:BCN9189
CAS No.:124709-39-3
- Drim-8(12)-ene-6β,7α,9α,11-tetraol
Catalog No.:BCN9190
CAS No.:2193060-24-9
- (8-Acetoxy-6-methyl-3,9-dimethylene-2-oxo-4,6a,7,8,9a,9b-hexahydro-3aH-azuleno[4,5-b]furan-4-yl) 3-acetoxy-2-hydroxy-2-methyl-butanoate
Catalog No.:BCN9191
CAS No.:
- Primeverose
Catalog No.:BCN9192
CAS No.:26531-85-1
- Aflavazole
Catalog No.:BCN9193
CAS No.:2043963-70-6
- Filicane-3β,4α,25-triol
Catalog No.:BCN9194
CAS No.:2361548-00-5
- Euglobal Ia1
Catalog No.:BCN9195
CAS No.:77844-93-0
- Herbarin
Catalog No.:BCN9196
CAS No.:36379-67-6
Effects of osmolytes on salt resistance of Halomonas socia CKY01 and identification of osmolytes-related genes by genome sequencing.[Pubmed:32653639]
J Biotechnol. 2020 Jul 9. pii: S0168-1656(20)30181-4.
Bacteria from the genus Halomonas hold promise in biotechnology as sources of salt-tolerant enzymes, biosurfactants, biopolymers, osmolytes, and as actors in bioremediation processes. In a previous work, we have identified Halomonas socia strain CKY01 having various hydrolase activities. Here, we aimed to study the survival strategies of marine bacteria. A deep genome sequencing study of H. socia CKY01 has revealed 4627 genes reaching 4,753,299 bp with 64% of GC content. This strain produced polyhydroxybutyrate (PHB) having one gene clusters having phaC and phasin, and it has several genes responsible for the uptake, synthesis, and transport of the osmolytes such as betaine, choline, Ectoine, carnitine, and proline in the bacterial genome. The addition of 60 mM glutamate, 60 mM proline and 60 mM Ectoine enhanced growth 300.8%, 294.2% and 235.0%, respectively, under 10% saline conditions. In particular, Ectoine and proline increased salt resistance and allowed cells to survive in more than 15% NaCl. By combining experimental and genome sequencing data, we have investigated the importance of osmolytes on the survival of this Halomonas strain.
The genome insights of Streptomyces lannensis T1317-0309 reveals actinomycin D production.[Pubmed:32641781]
J Antibiot (Tokyo). 2020 Jul 9. pii: 10.1038/s41429-020-0343-0.
The members of Streptomyces have been identified as a major source of antimicrobial agents with broad spectrum. This study is mainly focused on bioactivity-guided isolation and characterization of bioactive molecule from strain Streptomyces sp. T1317-0309 and its whole-genome sequence analysis for possible isolation of novel natural products. Strain Streptomyces sp. T1317-0309 showed 100% sequence similarity with strain Streptomyces lannensis TA4-8(T) consisting 10, 453,255 bp of genome with 5 scaffolds and 69.9 mol% G + C content. The genome analyses revealed a total of 17 putative biosynthetic gene clusters (BGCs) responsible for various secondary metabolites including actinomycin, bacteriocin, Ectoine, melanin, terpene, siderophore, betalactone, NRPS, T2PKS, and T3PKS. The BGC and bioactivity-guided purification of ethyl acetate extract of strain T1317-0309 showed the great potency of antimicrobial activities against various gram-positive multi-drug resistant human pathogens including MRSA. The BGC-predicted bioactive secondary metabolite was identified by various NMR analyses and confirmed as actinomycin D. In addition, this study reveals the first genome study of Streptomyces lannensis as a novel source for actinomycin D.
Role of N,N-dimethylglycine and its catabolism to sarcosine in Chromohalobacter salexigens DSM 3043.[Pubmed:32631860]
Appl Environ Microbiol. 2020 Jul 6. pii: AEM.01186-20.
Chromohalobacter salexigens DSM 3043 can grow on N,N-dimethylglycine (DMG) as sole C, N and energy source, and utilize sarcosine as sole N source under aerobic condition. However, little is known about the genes and enzymes involved in the conversion of DMG to sarcosine in this strain. In the present study, gene disruption and complementation assays indicated that the csal_0990, csal_0991, csal_0992 and csal_0993 genes are responsible for DMG degradation to sarcosine. The csal_0990 gene heterologously expressed in Escherichia coli was proved to encode an unusual DMG dehydrogenase (DMGDH). The enzyme, existing as a monomer of 79 kDa with a non-covalently bound flavin adenine dinucleotide, could utilize both DMG and sarcosine as substrates and exhibit dual coenzyme specificity preferring NAD(+) to NADP(+) The optimum pH and temperature of enzyme activity were determined to be 7.0 and 60 degrees C, respectively. Kinetic parameters of the enzyme toward its substrates were determined accordingly. Under high salinity conditions, the presence of DMG inhibited growth of the wild-type and induced the production and accumulation of trehalose and glucosylglycerate intracellularly. Moreover, exogenous addition of DMG could significantly improve the growth rates of the four DMG(-) mutants (Deltacsal_0990, Deltacsal_0991, Deltacsal_0992 and Deltacsal_0993) incubated at 37 degrees C in S-M63 synthetic medium with sarcosine as sole N source. (13)C-NMR experiments revealed that not only Ectoine, glutamate and Ngamma-acetyl-2,4-diaminobutyrate, but also glycine betaine (GB), DMG, sarcosine, trehalose, and glucosylglycerate are accumulated intracellularly in the four mutants.IMPORTANCE Although DMGDH activity was detected in cell extracts of microorganisms, the genes encoding microbial DMGDHs have not been determined until now. In addition, the physiological role of DMG in moderate halophile has never been investigated. In this study, we identified the genes involved in DMG degradation to sarcosine, characterized an unusual DMGDH, and investigated the role of DMG in C. salexigens DSM 3043 and its mutants. Our results suggested that the conversion of DMG to sarcosine is accompanied by intramolecular delivery of electrons in DMGDH and intermolecular electron transfer between DMGDH and other electron acceptors. Moreover, an unidentified methyltransferase catalyzing the production of GB from DMG but sharing no homology with the reported sarcosine DMG methyltransferases was predicted to be present in the cells. The results in this study expand our understanding on the physiological role of DMG and its catabolism to sarcosine in C. salexigens.
Raman Spectroscopic Signature of Ectoine Conformations in Bulk Solution and Crystalline State.[Pubmed:32628316]
Chemphyschem. 2020 Jul 6.
Recent crystallographic results revealed conformational changes of zwitterionic Ectoine upon hydration. By means of confocal Raman spectroscopy and density functional theory calculations, we present a detailed study of this transformation process as part of a Fermi resonance analysis. The corresponding findings highlight that all resonant couplings are lifted upon exposure to water vapor as a consequence of molecular binding processes. The importance of the involved molecular groups for water binding and conformational changes upon hydration is discussed. Our approach further shows that the underlying rapid process can be reversed by carbon dioxide saturated atmospheres. For the first time, we also confirm that the conformational state of Ectoine in aqueous bulk solution coincides with crystalline Ectoine in its dihydrate state, thereby highlighting the important role of a few bound water molecules.
Rational flux-tuning of Halomonas bluephagenesis for co-production of bioplastic PHB and ectoine.[Pubmed:32620759]
Nat Commun. 2020 Jul 3;11(1):3313.
Ectoine, a compatible solute synthesized by many halophiles for hypersalinity resistance, has been successfully produced by metabolically engineered Halomonas bluephagenesis, which is a bioplastic poly(3-hydroxybutyrate) producer allowing open unsterile and continuous conditions. Here we report a de novo synthesis pathway for Ectoine constructed into the chromosome of H. bluephagenesis utilizing two inducible systems, which serve to fine-tune the transcription levels of three clusters related to Ectoine synthesis, including ectABC, lysC and asd based on a GFP-mediated transcriptional tuning approach. Combined with bypasses deletion, the resulting recombinant H. bluephagenesis TD-ADEL-58 is able to produce 28 g L(-1) Ectoine during a 28 h fed-batch growth process. Co-production of Ectoine and PHB is achieved to 8 g L(-1) Ectoine and 32 g L(-1) dry cell mass containing 75% PHB after a 44 h growth. H. bluephagenesis demonstrates to be a suitable co-production chassis for polyhydroxyalkanoates and non-polymer chemicals such as Ectoine.
A Metabolomics Exploration of the Sexual Phase in the Marine Diatom Pseudo-nitzschia multistriata.[Pubmed:32545923]
Mar Drugs. 2020 Jun 14;18(6). pii: md18060313.
Pseudo-nitzschia multistriata is a planktonic marine diatom with a diplontic life cycle comprising a short sexual phase, during which gametes are produced following the encounter of two diploid cells of opposite mating type (MT). Gene expression studies have highlighted the presence of substantial changes occurring at the onset of sexual reproduction. Herein, we have hypothesized that the amount and nature of cellular metabolites varies along the mating process. To capture the metabolome of Pseudo-nitzschia multistriata at different harvesting times in an unbiased manner, we undertook an untargeted metabolomics approach based on liquid chromatography-tandem mass spectrometry. Using three different extraction steps, the method revealed pronounced differences in the metabolic profiles between control cells in the vegetative phase (MT+ and MT-) and mixed strains of opposite MTs (cross) undergoing sexual reproduction. Of the 2408 high-quality features obtained, 70 known metabolites could be identified based on in-house libraries and online databases; additional 46 features could be classified by molecular networking of tandem mass spectra. The reduction of phytol detected in the cross can be linked to the general downregulation of photosynthesis during sexual reproduction observed elsewhere. Moreover, the role of highly regulated compounds such as 7-dehydrodesmosterol, whose changes in abundance were the highest in the experiment, oleamide, Ectoine, or trigonelline is discussed.
Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics.[Pubmed:32486036]
Molecules. 2020 May 29;25(11). pii: molecules25112536.
The cosmetic industry is among the fastest growing industries in the last decade. As the beauty concepts have been revolutionized, many terms have been coined to accompany the innovation of this industry, since the beauty products are not just confined to those that are applied to protect and enhance the appearance of the human body. Consequently, the terms such as cosmeceuticals and nutricosmetics have emerged to give a notion of the health benefits of the products that create the beauty from inside to outside. In the past years, natural products-based cosmeceuticals have gained a huge amount of attention not only from researchers but also from the public due to the general belief that they are harmless. Notably, in recent years, the demand for cosmeceuticals from the marine resources has been exponentially on the rise due to their unique chemical and biological properties that are not found in terrestrial resources. Therefore, the present review addresses the importance of marine-derived compounds, stressing new chemical entities with cosmeceutical potential from the marine natural resources and their mechanisms of action by which these compounds exert on the body functions as well as their related health benefits. Marine environments are the most important reservoir of biodiversity that provide biologically active substances whose potential is still to be discovered for application as pharmaceuticals, nutraceuticals, and cosmeceuticals. Marine organisms are not only an important renewable source of valuable bulk compounds used in cosmetic industry such as agar and carrageenan, which are used as gelling and thickening agents to increase the viscosity of cosmetic formulations, but also of small molecules such as Ectoine (to promote skin hydration), trichodin A (to prevent product alteration caused by microbial contamination), and mytiloxanthin (as a coloring agent). Marine-derived molecules can also function as active ingredients, being the main compounds that determine the function of cosmeceuticals such as anti-tyrosinase (kojic acid), antiacne (sargafuran), whitening (chrysophanol), UV protection (scytonemin, mycosporine-like amino acids (MAAs)), antioxidants, and anti-wrinkle (astaxanthin and PUFAs).
Salt tolerance of nitrate reductase in Halomonas sp. B01.[Pubmed:32483684]
Folia Microbiol (Praha). 2020 Jun 1. pii: 10.1007/s12223-020-00801-9.
A systematic study on the lack of dissimilatory nitrate reductase (NAR) properties in Halomonas strains had been reported so far. The effects of different factors on Halomonas sp. B01 NAR activity were investigated. The salt tolerance of NAR was characterized. The denitrification process under high salt conditions was reported. Halomonas sp. B01 expressed membrane-bound NAR under induced culture by nitrate. The optimum pH of the enzyme reaction system was 8, and the optimum temperature was 30 degrees C. The mRNA expression abundance of narH in NAR encoding gene was highest in the 60 g/L NaCl inducing matrix. The NaCl concentration of optimum growth and induction of NAR were both 60 g/L. The Ectoine added to the NAR vitro enzyme reaction system could maintain NAR activity under high NaCl concentration. In the range of 0-60 g/L NaCl, the NAR activity was stable at 17.7 (+/- 0.3) U/mg. The denitrification was performed by Halomonas sp. B01 at 60 g/L NaCl, and the denitrification rate reached 97.1% at 24 h. This study reveals for the first time the NAR properties of Halomonas strains, which provides a theoretical and technical basis for the nitrogen removal of high-salt nitrogenous wastewater using this strain.
Complete Genome of Vibrio neocaledonicus CGJ02-2, An active Compounds Producing Bacterium Isolated from South China Sea.[Pubmed:32458061]
Curr Microbiol. 2020 May 26. pii: 10.1007/s00284-020-02047-7.
Strain CGJ02-2 was isolated from the coral reefs in South China sea and deposited in South China Sea Institute of Oceanology, Chinese Academy of Sciences. Active compounds including indole, rho-hydroxybenzaldehyde were isolated from this strain. To explore the biosynthetic way of these compounds and search gene clusters, the complete genome of this strain was sequenced by Single Molecule, Real-Time (SMRT) technology. It was de novo assembled to two circular chromosomes of 3,400,283 bp with GC% 44.77 and 1,845,572 bp with GC% 44.59 respectively and classified as Vibrio alginolyticus. In silico phenotype features of Vibrio alginolyticus CGJ02-2 were also analyzed. The biosynthetic pathway of rho-hydroxybenzaldehyde and indole in this strain were postulated. Gene clusters of four secondary metabolites including bacteriocin, Ectoine, siderophore, arylpolyene were identified. This study provides helpful information for further utilizing Vibrio alginolyticus CGJ02-2 as a source of valuable bioactive compounds.
Degradation of the microbial stress protectants and chemical chaperones ectoine and hydroxyectoine by a bacterial hydrolase-deacetylase complex.[Pubmed:32404365]
J Biol Chem. 2020 Jul 3;295(27):9087-9104.
When faced with increased osmolarity in the environment, many bacterial cells accumulate the compatible solute Ectoine and its derivative 5-hydroxyEctoine. Both compounds are not only potent osmostress protectants, but also serve as effective chemical chaperones stabilizing protein functionality. Ectoines are energy-rich nitrogen and carbon sources that have an ecological impact that shapes microbial communities. Although the biochemistry of Ectoine and 5-hydroxyEctoine biosynthesis is well understood, our understanding of their catabolism is only rudimentary. Here, we combined biochemical and structural approaches to unravel the core of Ectoine and 5-hydroxy-Ectoine catabolisms. We show that a conserved enzyme bimodule consisting of the EutD Ectoine/5-hydroxyEctoine hydrolase and the EutE deacetylase degrades both Ectoines. We determined the high-resolution crystal structures of both enzymes, derived from the salt-tolerant bacteria Ruegeria pomeroyi and Halomonas elongata These structures, either in their apo-forms or in forms capturing substrates or intermediates, provided detailed insights into the catalytic cores of the EutD and EutE enzymes. The combined biochemical and structural results indicate that the EutD homodimer opens the pyrimidine ring of Ectoine through an unusual covalent intermediate, N-alpha-2 acetyl-l-2,4-diaminobutyrate (alpha-ADABA). We found that alpha-ADABA is then deacetylated by the zinc-dependent EutE monomer into diaminobutyric acid (DABA), which is further catabolized to l-aspartate. We observed that the EutD-EutE bimodule synthesizes exclusively the alpha-, but not the gamma-isomers of ADABA or hydroxy-ADABA. Of note, alpha-ADABA is known to induce the MocR/GabR-type repressor EnuR, which controls the expression of many Ectoine catabolic genes clusters. We conclude that hydroxy-alpha-ADABA might serve a similar function.
Primary purification of intracellular Halomonas salina ectoine using ionic liquids-based aqueous biphasic system.[Pubmed:32389469]
J Biosci Bioeng. 2020 Aug;130(2):200-204.
Ectoine is a zwitterionic amino acid derivative that can be naturally sourced from halophilic microorganisms. The increasing demands of Ectoine in various industries have urged the researches on the cost-effective approaches on production of Ectoine. Ionic liquids-based aqueous biphasic system (ILABS) was applied to recover Halomonas salina Ectoine from cells hydrolysate. The 1-butyl-3-methylimidazolium tetrafluoroborate (Bmim)BF4 was used in the ILABS and the recovery efficiency of ILABS to recover Ectoine from H. salina cells lysate was evaluated by determining the effects of phase composition; pHs; crude loading and additional neutral salt (NaCl). The hydrophilic Ectoine was targeted to partition to the hydrophilic salt-rich phase. A total yield (YB) of 96.32% +/- 1.08 of Ectoine was obtained with ILABS of phase composition of 20% (w/w) (Bmim)BF4 and 30% (w/w) sulfate salts; system pH of 5.5 when the 20% (w/w) of crude feedstock was applied to the ILABS. There was no significant enhancement on the Ectoine recovery efficiency using the ILABS when NaCl was added, therefore the ILABS composition without the additional neutral salt was recommended for the primary purification of Ectoine. Partition coefficient (KE) of 30.80 +/- 0.42, purity (PE) of 95.82% and enrichment factor (Ef) of 1.92 were recorded with the optimum (Bmim)BF4/sulfate ILABS. These findings have provided an insight on the feasibility of recovery of intracellular biomolecules using the green solvent-based aqueous system in one single-step operation.
Exploring the additive bio-agent impacts upon ectoine production by Halomonas salina DSM5928(T) using corn steep liquor and soybean hydrolysate as nutrient supplement.[Pubmed:32370929]
J Biosci Bioeng. 2020 Aug;130(2):195-199.
Ectoine production using inexpensive and renewable biomass resources has attracted great interest among the researchers due to the low yields of Ectoine in current fermentation approaches that complicate the large-scale production of Ectoine. In this study, Ectoine was produced from corn steep liquor (CSL) and soybean hydrolysate (SH) in replacement to yeast extract as the nitrogen sources for the fermentation process. To enhance the bacterial growth and Ectoine production, biotin was added to the Halomonas salina fermentation media. In addition, the effects addition of surfactants such as Tween 80 and saponin on the Ectoine production were also investigated. Results showed that both the CSL and SH can be used as the nitrogen source substitutes in the fermentation media. Higher amount of Ectoine (1781.9 mg L(-1)) was produced in shake flask culture with SH-containing media as compared to CSL-containing media. A total of 2537.0 mg L(-1) of Ectoine was produced at pH 7 when SH-containing media was applied in the 2 L batch fermentation. Moreover, highest amount of Ectoine (1802.0 mg L(-1)) was recorded in the SH-containing shake flask culture with addition of 0.2 mum mL(-1) biotin. This study demonstrated the efficacy of industrial waste as the nutrient supplement for the fermentation of Ectoine production.
Ectoine degradation pathway in halotolerant methylotrophs.[Pubmed:32353000]
PLoS One. 2020 Apr 30;15(4):e0232244.
BACKGROUND: Microorganisms living in saline environments are forced to regulate turgor via the synthesis of organic osmoprotective compounds. Microbial adaptation to fluctuations in external salinity includes degradation of compatible solutes. Here we have examined the biochemical pathway of degradation of the cyclic imino acid Ectoine, the major osmoprotector in halotolerant methane-utilizing bacteria. METHODS: The BLAST search of the genes involved in Ectoine degradation in the halotolerant methanotroph Methylotuvimicrobium alcaliphilum 20Z was performed with the reference sequences of Halomonas elongata. The genes for the key enzymes of the pathway were disrupted by insertion mutagenesis and the cellular metabolites in the methanol extracts of mutant cells were analyzed by HPLC. The doeA gene from Mm. alcaliphilum 20Z was heterologously expressed in Escherichia coli to identify the product of Ectoine hydrolysis catalyzed by Ectoine hydrolase DoeA. RESULTS: We have shown that the halotolerant methanotroph Mm. alcaliphilum 20Z possesses the doeBDAC gene cluster coding for putative Ectoine hydrolase (DoeA), Nalpha-acetyl-L-2,4-diaminobutyrate deacetylase (DoeB), diaminobutyrate transaminase (DoeD) and aspartate-semialdehyde dehydrogenase (DoeC). The deletion of the doeA gene resulted in accumulation of the higher level of Ectoine compared to the wild type strain. Ngamma-acetyl-L-2,4-diaminobutyrate (Ngamma-acetyl-DAB), a substrate for Ectoine synthase, was found in the cytoplasm of the wild type strain. Nalpha-acetyl-L-2,4-diaminobutyrate (Nalpha-acetyl-DAB), a substrate for the DoeB enzyme, appeared in the cells as a result of exposure of the doeB mutant to low osmotic pressure. The genes for the enzymes involved in Ectoine degradation were found in all aerobic methylotrophs capable of Ectoine biosynthesis. These results provide the first evidence for the in vivo operation of the Ectoine degradation pathway in methanotrophs and thus expand our understanding of the regulation mechanisms of bacterial osmoadaptation. CONCLUSIONS: During adaptation to the changes in external osmolarity, halophilic and halotolerant methylotrophs cleave Ectoine, thereby entering the carbon and nitrogen of the compatible solute to the central metabolic pathways. The biochemical route of Ectoine degradation in the halotolerant methanotroph Mm. alcaliphilum 20Z is similar to that in heterotrophic halophiles. We have shown that Ectoine hydrolase DoeA in this methanotroph hydrolyzes Ectoine with the formation of the only isomer: Nalpha-acetyl-DAB. All aerobic methylotrophs capable of Ectoine biosynthesis harbor the genetic determinants for Ectoine degradation.


