EGTACAS# 67-42-5 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 67-42-5 | SDF | Download SDF |
| PubChem ID | 6207 | Appearance | Powder |
| Formula | C14H24N2O10 | M.Wt | 380.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1M NaOH | ||
| Chemical Name | 2-[2-[2-[2-[bis(carboxymethyl)amino]ethoxy]ethoxy]ethyl-(carboxymethyl)amino]acetic acid | ||
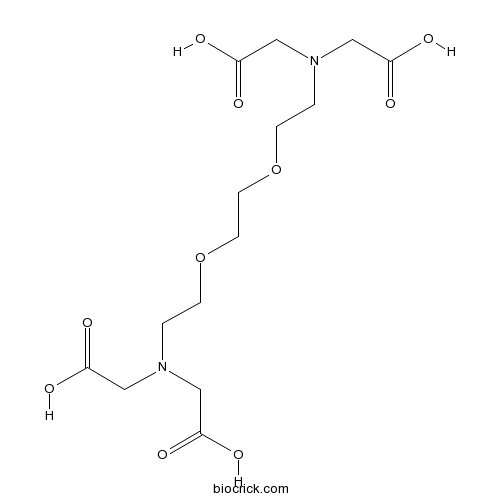
| SMILES | C(COCCOCCN(CC(=O)O)CC(=O)O)N(CC(=O)O)CC(=O)O | ||
| Standard InChIKey | DEFVIWRASFVYLL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H24N2O10/c17-11(18)7-15(8-12(19)20)1-3-25-5-6-26-4-2-16(9-13(21)22)10-14(23)24/h1-10H2,(H,17,18)(H,19,20)(H,21,22)(H,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Calcium chelator; protects against cell death caused by nitric oxide-induced calcium influx into nerve cells. |

EGTA Dilution Calculator

EGTA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6292 mL | 13.1458 mL | 26.2916 mL | 52.5831 mL | 65.7289 mL |
| 5 mM | 0.5258 mL | 2.6292 mL | 5.2583 mL | 10.5166 mL | 13.1458 mL |
| 10 mM | 0.2629 mL | 1.3146 mL | 2.6292 mL | 5.2583 mL | 6.5729 mL |
| 50 mM | 0.0526 mL | 0.2629 mL | 0.5258 mL | 1.0517 mL | 1.3146 mL |
| 100 mM | 0.0263 mL | 0.1315 mL | 0.2629 mL | 0.5258 mL | 0.6573 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Thiamine hydrochloride
Catalog No.:BCN2225
CAS No.:67-03-8
- Tianeptine
Catalog No.:BCC1999
CAS No.:66981-73-5
- Beta-Belladonnine
Catalog No.:BCN1893
CAS No.:6696-63-5
- α-Conotoxin PIA
Catalog No.:BCC5976
CAS No.:669050-68-4
- 1,2-O-Isopropylidene-beta-D-fructopyranose
Catalog No.:BCN1383
CAS No.:66900-93-4
- Talniflumate
Catalog No.:BCC7391
CAS No.:66898-62-2
- H-D-Leu-OBzl.HCl
Catalog No.:BCC2682
CAS No.:66866-69-1
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- LDN 57444
Catalog No.:BCC2087
CAS No.:668467-91-2
- Linagliptin (BI-1356)
Catalog No.:BCC2110
CAS No.:668270-12-0
- Magnoflorine chloride
Catalog No.:BCN2405
CAS No.:6681-18-1
- Jatrorrhizine chloride
Catalog No.:BCN4956
CAS No.:6681-15-8
- Furazolidone
Catalog No.:BCC8988
CAS No.:67-45-8
- 5-Hydroxymethylfurfural
Catalog No.:BCN4226
CAS No.:67-47-0
- Fluocinolone Acetonide
Catalog No.:BCC4906
CAS No.:67-73-2
- Dicyclomine HCl
Catalog No.:BCC3762
CAS No.:67-92-5
- Vitamin D3
Catalog No.:BCN2186
CAS No.:67-97-0
- Gliotoxin
Catalog No.:BCN3894
CAS No.:67-99-2
- Anthriscusin
Catalog No.:BCN3533
CAS No.:67008-16-6
- Methyl isovanillate
Catalog No.:BCN7960
CAS No.:6702-50-7
- 3,4-Dihydroxy-2-methoxyxanthone
Catalog No.:BCN7631
CAS No.:6702-55-2
- Crenolanib (CP-868596)
Catalog No.:BCC3671
CAS No.:670220-88-9
- Ohchinin
Catalog No.:BCN4601
CAS No.:67023-80-7
- Ohchinin acetate
Catalog No.:BCN4220
CAS No.:67023-81-8
A novel explanation for observed CaMKII dynamics in dendritic spines with added EGTA or BAPTA.[Pubmed:25692602]
Biophys J. 2015 Feb 17;108(4):975-985.
We present a simplified reaction network in a single well-mixed volume that captures the general features of CaMKII dynamics observed during both synaptic input and spine depolarization. Our model can also account for the greater-than-control CaMKII activation observed with added EGTA during depolarization. Calcium input currents are modeled after experimental observations, and existing models of calmodulin and CaMKII autophosphorylation are used. After calibration against CaMKII activation data in the absence of chelators, CaMKII activation dynamics due to synaptic input via n-methyl-d-aspartate receptors are qualitatively accounted for in the presence of the chelators EGTA and BAPTA without additional adjustments to the model. To account for CaMKII activation dynamics during spine depolarization with added EGTA or BAPTA, the model invokes the modulation of CaV2.3 (R-type) voltage-dependent calcium channel (VDCC) currents observed in the presence of EGTA or BAPTA. To our knowledge, this is a novel explanation for the increased CaMKII activation seen in dendritic spines with added EGTA, and suggests that differential modulation of VDCCs by EGTA and BAPTA offers an alternative or complementary explanation for other experimental results in which addition of EGTA or BAPTA produces different effects. Our results also show that a simplified reaction network in a single, well-mixed compartment is sufficient to account for the general features of observed CaMKII dynamics.
The integrin alphaL leg region controls the Mg/EGTA mediated activation of LFA-1.[Pubmed:25640842]
Biochem Biophys Res Commun. 2015 Mar 6;458(2):251-5.
We have shown that Mg/EGTA (5 mM Mg(2+) and 1.5 mM EGTA) could effectively promote the adhesion of integrin alphaLbeta2 to its ligand ICAM-1 but could not promote that of the alphaMbeta2 to denatured BSA. In order to determine the structural differences between alphaL and alphaM that specifically contribute to Mg/EGTA sensitivity, a series of alphaL/alphaM chimeras were constructed. Our results showed that alphaLbeta2 with alphaM calf-1 domain completely lost the response to Mg/EGTA activation. In the reverse experiment, alphaMbeta2 would require the presence of both the alphaL calf-1 and calf-2 domain to initiate the Mg/EGTA sensitivity.
Ca(2+) Binding and Transport Studied with Ca(2+)/EGTA Buffers and (45)Ca(2+).[Pubmed:26695038]
Methods Mol Biol. 2016;1377:261-6.
The chapter describes procedures useful for determination of Ca(2+) binding by membranous Ca(2+)-ATPase based on the correction for the removal of Ca(2+) present in a non-bound state in the suspension medium. This is done by a filtration procedure that retains the membranous material on the Millipore filters. With suitable sucking devices it is possible to gently remove without dehydration nearly all medium from the Ca(2+) containing membranes, except that required for wetting of the filters on which they are deposited. Correction for this effect can be done with a double-filter where the radioactive content of the lower (protein-free) filter is subtracted from that present in the upper filter for calculation of Ca(2+) binding. This methodology can be used to study the effect of inhibitors on Ca(2+) binding and -transport, and with Ca(2+)/EGTA buffers to explore the Ca(2+) binding affinities and cooperative aspects of the two transport sites.
Fast adsorption of Cd(2)(+) and Pb(2)(+) by EGTA dianhydride (EGTAD) modified ramie fiber.[Pubmed:25181330]
J Colloid Interface Sci. 2014 Nov 15;434:152-8.
In this study, the removal of Cd(2+) and Pb(2+) from aqueous solutions was investigated using a novel chelating material. The first part described the synthesis of ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid dianhydride (EGTAD), mercerization treatment of ramie fiber (MRF), and the MRF was then reacted with EGTAD to prepare the new material (ERF). The obtained material was characterized by weight gain, SEM, FTIR, and elemental analysis. The results of FTIR and elemental analysis confirmed that ester bond, carboxyl and amine groups were introduced onto ERF. The adsorption capacity of metals on ERF was evaluated at different contact times, pH values, initial metal concentrations, and temperatures in the second part. The adsorption equilibrium was reached within 5 min for Cd(2+) and Pb(2+). Adsorption isotherm could be well fitted by the Langmuir model, and the maximum adsorption capacities were 159.11 and 273.78 mg g(-1) for Cd(2+) and Pb(2+) at 298 K, respectively. Thermodynamic analysis showed that the adsorption process was spontaneous and endothermic. The molar ratio of adsorbed cation to grafted EGTA is close to 1.8:1, which confirmed that the adsorption was chemical process involving both surface chelation reaction and ion exchange. In addition, the absorbent was successfully regenerated using HCl and ultrasonic treatment.
Role of calcium in nitric oxide-induced cytotoxicity: EGTA protects mouse oligodendrocytes.[Pubmed:11169622]
J Neurosci Res. 2001 Jan 15;63(2):124-35.
Active nitrogen species are overproduced in inflammatory brain lesions in multiple sclerosis (MS) and experimental allergic encephalomyelitis (EAE). NO has been shown to mediate the death of oligodendrocytes (OLs), a primary target of damage in MS. To develop strategies to protect OLs, we examined the mechanisms of cytotoxicity of two NO donors, S-nitroso-N-acetyl-penicillamine (SNAP) and sodium nitroprusside (SNP) on mature mouse OLs. Nitrosonium ion (NO+) rather than NO. mediates damage with both SNAP and SNP, as shown by significant protection with hemoglobin (HbO2), but not with the NO. scavenger PTIO. SNAP and SNP differ in time course and mechanisms of killing OLs. With SNAP, OL death is delayed for at least 6 hr, but with SNP, OL death is continuous over 18 hr with no delay. Relative to NO release, SNP is more toxic than SNAP, due to synergism of NO with cyanide released by SNP. SNAP elicits a Ca2+ influx in over half of the OLs within min. Further, OL death due to NO release from SNAP is Ca2+-dependent, because the Ca2+ chelator EGTA protects OLs from killing by SNAP, and also from killing by the NONOates NOC-9 and NOC-18, which spontaneously release NO. SNP does not elicit a Ca2+ influx, and EGTA is not protective. In comparison to the N20.1 OL cell line (Boullerne et al., [1999] J. Neurochem. 72:1050-1060), mature OLs are (1) more sensitive to SNAP, (2) much more resistant to SNP, (3) sensitive to cyanide, but not iron, and (4) exhibit a Ca2+ influx and EGTA protection in response to NO generated by SNAP.


