DihydrochelerythrineCAS# 6880-91-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6880-91-7 | SDF | Download SDF |
| PubChem ID | 485077 | Appearance | Powder |
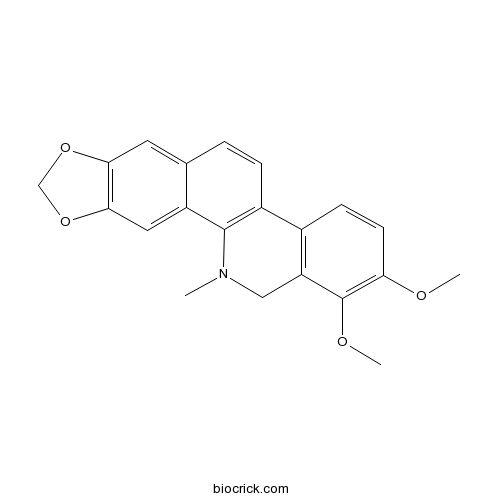
| Formula | C21H19NO4 | M.Wt | 349.38 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (143.11 mM; Need ultrasonic and warming) | ||
| Chemical Name | 1,2-dimethoxy-12-methyl-13H-[1,3]benzodioxolo[5,6-c]phenanthridine | ||
| SMILES | CN1CC2=C(C=CC(=C2OC)OC)C3=C1C4=CC5=C(C=C4C=C3)OCO5 | ||
| Standard InChIKey | ALZAZMCIBRHMFF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H19NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-9H,10-11H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dihydrochelerythrine has antifungal activity against pathogenic plant fungi; it shows antiparasitic efficacy against Ichthyophthirius multifiliis in richadsin, it has potential application in the therapy of serious infection caused by I. multifiliis. Dihydrochelerythrine affects cell cycle distribution, activates mitochondrial apoptotic pathway, and induces apoptosis and necrosis in HL-60 cells. |
| Targets | Antifection | Caspase |
| In vitro | Inhibitory activity of dihydrosanguinarine and dihydrochelerythrine against phytopathogenic fungi.[Pubmed: 21500094]Nat Prod Res. 2011 Jul;25(11):1082-9.The antifungal activities of dihydrosanguinarine and Dihydrochelerythrine, isolated from the leaves of Macleaya microcarpa, were evaluated on 12 plant pathogenic fungi; the two compounds exhibited the highest antifungal activity against Botrytis cinerea Pers.
|
| In vivo | Antiparasitic efficacy of dihydrosanguinarine and dihydrochelerythrine from Macleaya microcarpa against Ichthyophthirius multifiliis in richadsin (Squaliobarbus curriculus).[Pubmed: 21813242]Vet Parasitol. 2011 Dec 29;183(1-2):8-13.Ichthyophthirius multifiliis is a holotrichous protozoan that invades the gills and skin surfaces of fish and can cause morbidity and high mortality in most species of freshwater fish worldwide.
The present study was undertaken to investigate the antiparasitic activity of crude extracts and pure compounds from the leaves of Macleaya microcarpa.
|
| Cell Research | Chelerythrine and dihydrochelerythrine induce G1 phase arrest and bimodal cell death in human leukemia HL-60 cells.[Pubmed: 18358694]Toxicol In Vitro. 2008 Jun;22(4):1008-17.A quaternary benzo[c]phenanthridine alkaloid chelerythrine displays a wide range of biological activities including cytotoxicity to normal and cancer cells. In contrast, less is known about the biological activity of Dihydrochelerythrine, a product of chelerythrine reduction.
|
| Structure Identification | J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Dec 15;941:17-24.Mass spectrometric investigation of chelerythrine and dihydrochelerythrine biotransformation patterns in human hepatocytes.[Pubmed: 24184831]The quaternary benzo[c]phenanthridine alkaloids (QBAs) are an important subgroup of plant secondary metabolites. Their main representatives, sanguinarine (SG) and chelerythrine (CHE), have pleiotropic biological effects and a wide spectrum of medicinal applications. The biotransformation of SG and CHE has only been partially studied while subsequent oxidative transformation of their dihydro derivates, the main metabolites, is practically unknown. The aim of this study was to characterize the biotransformation of CHE and Dihydrochelerythrine (DHCHE) in detail, with respect to their more extensive biotransformation than SG.
|

Dihydrochelerythrine Dilution Calculator

Dihydrochelerythrine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8622 mL | 14.3111 mL | 28.6221 mL | 57.2443 mL | 71.5553 mL |
| 5 mM | 0.5724 mL | 2.8622 mL | 5.7244 mL | 11.4489 mL | 14.3111 mL |
| 10 mM | 0.2862 mL | 1.4311 mL | 2.8622 mL | 5.7244 mL | 7.1555 mL |
| 50 mM | 0.0572 mL | 0.2862 mL | 0.5724 mL | 1.1449 mL | 1.4311 mL |
| 100 mM | 0.0286 mL | 0.1431 mL | 0.2862 mL | 0.5724 mL | 0.7156 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Norfluorocurarine
Catalog No.:BCN4811
CAS No.:6880-54-2
- 6-Acetyl-2,2-dimethylchroman-4-one
Catalog No.:BCN4248
CAS No.:68799-41-7
- 4-Oxobedfordiaic acid
Catalog No.:BCN4247
CAS No.:68799-38-2
- Dipotassium glycyrrhizinate
Catalog No.:BCN8487
CAS No.:68797-35-3
- Dehydroeburicoic acid
Catalog No.:BCN3646
CAS No.:6879-05-6
- Tuberstemonine
Catalog No.:BCN4986
CAS No.:6879-01-2
- Triclabendazole
Catalog No.:BCC4742
CAS No.:68786-66-3
- 1beta-Hydroxyalantolactone
Catalog No.:BCN3508
CAS No.:68776-47-6
- 12-Hydroxy-2,3-dihydroeuparin
Catalog No.:BCN8115
CAS No.:68776-42-1
- Corynoxine
Catalog No.:BCN2364
CAS No.:6877-32-3
- Loxoprofen
Catalog No.:BCC5191
CAS No.:68767-14-6
- (±)-Pinocembrin
Catalog No.:BCN3537
CAS No.:68745-38-0
- Sophoridine
Catalog No.:BCN4249
CAS No.:6882-68-4
- Sempervirine
Catalog No.:BCN4250
CAS No.:6882-99-1
- Retusamine
Catalog No.:BCN2122
CAS No.:6883-16-5
- D-Prolinol(oil)
Catalog No.:BCC2708
CAS No.:68832-13-3
- 4-Epi-isoinuviscolide
Catalog No.:BCN4251
CAS No.:68832-39-3
- IWP 12
Catalog No.:BCC5622
CAS No.:688353-45-9
- Xanthohumol I
Catalog No.:BCN8016
CAS No.:688360-06-7
- Xanthohumol L
Catalog No.:BCN8017
CAS No.:688360-15-8
- Astemizole
Catalog No.:BCC7691
CAS No.:68844-77-9
- Boc-D-Tyr(Me)-OH
Catalog No.:BCC2597
CAS No.:68856-96-2
- Fmoc-Val-OH
Catalog No.:BCC3570
CAS No.:68858-20-8
- HMP Linker
Catalog No.:BCC2832
CAS No.:68858-21-9
Mass spectrometric investigation of chelerythrine and dihydrochelerythrine biotransformation patterns in human hepatocytes.[Pubmed:24184831]
J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Dec 15;941:17-24.
The quaternary benzo[c]phenanthridine alkaloids (QBAs) are an important subgroup of plant secondary metabolites. Their main representatives, sanguinarine (SG) and chelerythrine (CHE), have pleiotropic biological effects and a wide spectrum of medicinal applications. The biotransformation of SG and CHE has only been partially studied while subsequent oxidative transformation of their dihydro derivates, the main metabolites, is practically unknown. The aim of this study was to characterize the biotransformation of CHE and Dihydrochelerythrine (DHCHE) in detail, with respect to their more extensive biotransformation than SG. Phase I as well as phase II biotransformation of both compounds was examined in human hepatocyte suspensions. Liquid chromatography with electrospray-quadrupole time-of-flight mass spectrometry (LC-ESI-QqTOF MS) was used for analysis of the metabolites. Using the LC-ESI-QqTOF MS method, we analyzed and then suggested the putative structures of 11 phase I and 5 phase II metabolites of CHE, and 11 phase I and 6 phase II metabolites of DHCHE. For the most abundant metabolites of CHE, DHCHE and O-demethylated DHCHE, their cytotoxicity on primary cultures of human hepatocytes was analyzed. Both metabolites were nontoxic up to 50muM concentration and this indicates decreasing toxic effects for CHE biotransformation products, i.e. DHCHE and O-demethylated DHCHE.
Inhibitory activity of dihydrosanguinarine and dihydrochelerythrine against phytopathogenic fungi.[Pubmed:21500094]
Nat Prod Res. 2011 Jul;25(11):1082-9.
The antifungal activities of dihydrosanguinarine and Dihydrochelerythrine, isolated from the leaves of Macleaya microcarpa, were evaluated on 12 plant pathogenic fungi; the two compounds exhibited the highest antifungal activity against Botrytis cinerea Pers. Among the 11 tested plant pathogenic fungi in vitro, the two compounds showed the highest antifungal activity against B. cinerea Pers, with 95.16% and 98.32% mycelial growth inhibition at 50 microg mL(-)(1), respectively. In addition, the two compounds inhibited spore germination in vitro in a concentration-dependent manner. They also showed potent protective and curative effects against Erysiphe graminis and B. cinerea in vivo. This is the first report on the antifungal activity of dihydrosanguinarine and Dihydrochelerythrine against pathogenic plant fungi.
Chelerythrine and dihydrochelerythrine induce G1 phase arrest and bimodal cell death in human leukemia HL-60 cells.[Pubmed:18358694]
Toxicol In Vitro. 2008 Jun;22(4):1008-17.
A quaternary benzo[c]phenanthridine alkaloid chelerythrine displays a wide range of biological activities including cytotoxicity to normal and cancer cells. In contrast, less is known about the biological activity of Dihydrochelerythrine, a product of chelerythrine reduction. We examined the cytotoxicity of chelerythrine and Dihydrochelerythrine in human promyelocytic leukemia HL-60 cells. After 4h of treatment, chelerythrine induced a dose-dependent decrease in the cell viability with IC50 of 2.6 microM as shown by MTT reduction assay. Dihydrochelerythrine appeared to be less cytotoxic since the viability of cells exposed to 20 microM Dihydrochelerythrine for 24h was reduced only to 53%. Decrease in the viability induced by both alkaloids was accompanied by apoptotic events including the dissipation of mitochondrial membrane potential, activation of caspase-9 and -3, and appearance of cells with sub-G1 DNA. Moreover, chelerythrine, but not Dihydrochelerythrine, elevated the activity of caspase-8. A dose-dependent induction of apoptosis and necrosis by chelerythrine and Dihydrochelerythrine was confirmed by annexin V/propidium iodide dual staining flow cytometry. Besides, both alkaloids were found to induce accumulation of HL-60 cells in G1 phase of the cell cycle. We conclude that both chelerythrine and Dihydrochelerythrine affect cell cycle distribution, activate mitochondrial apoptotic pathway, and induce apoptosis and necrosis in HL-60 cells.
Antiparasitic efficacy of dihydrosanguinarine and dihydrochelerythrine from Macleaya microcarpa against Ichthyophthirius multifiliis in richadsin (Squaliobarbus curriculus).[Pubmed:21813242]
Vet Parasitol. 2011 Dec 29;183(1-2):8-13.
Ichthyophthirius multifiliis is a holotrichous protozoan that invades the gills and skin surfaces of fish and can cause morbidity and high mortality in most species of freshwater fish worldwide. The present study was undertaken to investigate the antiparasitic activity of crude extracts and pure compounds from the leaves of Macleaya microcarpa. The chloroform extract showed a promising antiparasitic activity against I. multifiliis. Based on these finding, the chloroform extract was fractionated on silica gel column chromatography in a bioactivity-guided isolation affording two compounds showing potent activity. The structures of the two compounds were elucidated as dihydrosanguinarine and Dihydrochelerythrine by hydrogen and carbon-13 nuclear magnetic resonance spectrum and electron ionization mass spectrometry. The in vivo tests revealed that dihydrosanguinarine and Dihydrochelerythrine were effective against I. multifiliis with median effective concentration (EC(50)) values of 5.18 and 9.43 mg/l, respectively. The acute toxicities (LC(50)) of dihydrosanguinarine and Dihydrochelerythrine for richadsin were 13.3 and 18.2mg/l, respectively. The overall results provided important information for the potential application of dihydrosanguinarine and Dihydrochelerythrine in the therapy of serious infection caused by I. multifiliis.


