DiclofenacCAS# 15307-86-5 |
- S0859
Catalog No.:BCC1914
CAS No.:1019331-10-2
- Sodium Channel inhibitor 1
Catalog No.:BCC1959
CAS No.:1198117-23-5
- Nav1.7 inhibitor
Catalog No.:BCC4191
CAS No.:1355631-24-1
- Riluzole
Catalog No.:BCC3849
CAS No.:1744-22-5
- Flecainide acetate
Catalog No.:BCC1578
CAS No.:54143-56-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15307-86-5 | SDF | Download SDF |
| PubChem ID | 3033 | Appearance | Powder |
| Formula | C14H11Cl2NO2 | M.Wt | 296.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 3.5 mg/mL (11.82 mM) *"≥" means soluble, but saturation unknown. | ||
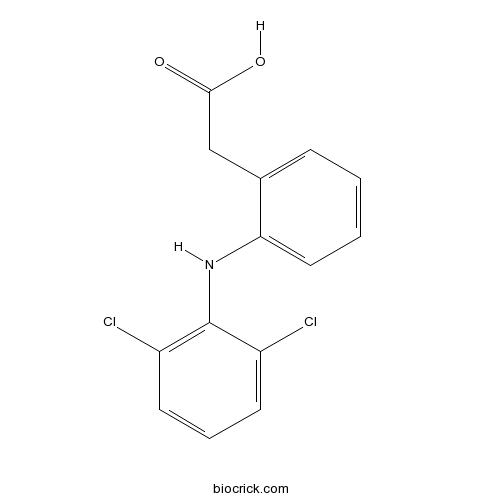
| Chemical Name | 2-[2-(2,6-dichloroanilino)phenyl]acetic acid | ||
| SMILES | C1=CC=C(C(=C1)CC(=O)O)NC2=C(C=CC=C2Cl)Cl | ||
| Standard InChIKey | DCOPUUMXTXDBNB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Diclofenac Dilution Calculator

Diclofenac Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3767 mL | 16.8833 mL | 33.7667 mL | 67.5333 mL | 84.4167 mL |
| 5 mM | 0.6753 mL | 3.3767 mL | 6.7533 mL | 13.5067 mL | 16.8833 mL |
| 10 mM | 0.3377 mL | 1.6883 mL | 3.3767 mL | 6.7533 mL | 8.4417 mL |
| 50 mM | 0.0675 mL | 0.3377 mL | 0.6753 mL | 1.3507 mL | 1.6883 mL |
| 100 mM | 0.0338 mL | 0.1688 mL | 0.3377 mL | 0.6753 mL | 0.8442 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Diclofenac is a non-selective COX inhibitor with IC50 of 60 and 220 nM for ovine COX-1 and -2, respectively. Target: COX Diclofenac is a nonsteroidal anti-inflammatory drug (NSAID) taken or applied to reduce inflammation and as an analgesic reducing pain in certain conditions. The primary mechanism responsible for its anti-inflammatory, antipyretic, and analgesic action is thought to be inhibition of prostaglandin synthesis by inhibition of cyclooxygenase (COX). It also appears to exhibit bacteriostatic activity by inhibiting bacterial DNA synthesis. Inhibition of COX also decreases prostaglandins in the epithelium of the stomach, making it more sensitive to corrosion by gastric acid. This is also the main side effect of diclofenac. Diclofenac has a low to moderate preference to block the COX2-isoenzyme and is said to have, therefore, a somewhat lower incidence of gastrointestinal complaints than noted with indomethacin and aspirin [1, 2].
References:
[1]. Dastidar, S.G., et al., The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis. Int J Antimicrob Agents, 2000. 14(3): p. 249-51.
[2]. Fowler, P.D., et al., Plasma and synovial fluid concentrations of diclofenac sodium and its major hydroxylated metabolites during long-term treatment of rheumatoid arthritis. Eur J Clin Pharmacol, 1983. 25(3): p. 389-94.
- Diclofenac Sodium
Catalog No.:BCC4439
CAS No.:15307-79-6
- SR 140333
Catalog No.:BCC6098
CAS No.:153050-21-6
- Precursor of cefcapene diisopropylanmine salt
Catalog No.:BCC9127
CAS No.:153012-37-4
- Serotonin HCl
Catalog No.:BCC4715
CAS No.:153-98-0
- H-D-Trp-OH
Catalog No.:BCC3117
CAS No.:153-94-6
- 2-Aminofluorene
Catalog No.:BCC8549
CAS No.:153-78-6
- Rutin
Catalog No.:BCN1684
CAS No.:153-18-4
- Guajadial F
Catalog No.:BCN6437
CAS No.:1529775-08-3
- Guajadial E
Catalog No.:BCN7754
CAS No.:1529775-06-1
- Guajadial D
Catalog No.:BCN7756
CAS No.:1529775-04-9
- Guajadial C
Catalog No.:BCN7755
CAS No.:1529775-02-7
- Dehydro-alpha-lapachone
Catalog No.:BCN1683
CAS No.:15297-92-4
- N-Methyltaxol C
Catalog No.:BCN7343
CAS No.:153083-53-5
- Thiamphenicol
Catalog No.:BCC4736
CAS No.:15318-45-3
- Taxayunnansin A
Catalog No.:BCN1685
CAS No.:153229-31-3
- Cilomilast
Catalog No.:BCC2283
CAS No.:153259-65-5
- ML355
Catalog No.:BCC8060
CAS No.:1532593-30-8
- 4,4'-Bis(2-benzoxazolyl)stilbene
Catalog No.:BCC8656
CAS No.:1533-45-5
- DFB
Catalog No.:BCC7130
CAS No.:15332-10-2
- SCR7
Catalog No.:BCC3978
CAS No.:1533426-72-0
- Ginkgolide K
Catalog No.:BCN8209
CAS No.:153355-70-5
- 7-Epi-docetaxel
Catalog No.:BCC5411
CAS No.:153381-68-1
- Taxol C
Catalog No.:BCN6941
CAS No.:153415-45-3
- Taxcultine
Catalog No.:BCN6948
CAS No.:153415-46-4
Diclofenac Does Not Reduce the Risk of Post-endoscopic Retrograde Cholangiopancreatography Pancreatitis in Low-Risk Units.[Pubmed:28374181]
J Gastrointest Surg. 2017 Aug;21(8):1270-1277.
BACKGROUND: Nonsteroidal anti-inflammatory drugs have an inhibitory role in pathogenesis of pancreatitis. Guidelines from the European Society of Gastrointestinal Endoscopy recommend routine rectal administration of 100 mg of Diclofenac or indomethacin immediately before or after ERCP for all patients without contraindications. AIMS: Our aim was to evaluate the effect of Diclofenac in preventing post-ERCP pancreatitis (PEP) in a high-volume, low-PEP-risk ERCP unit. METHODS: The rate and severity of PEP were compared in groups of 1000 historical controls prior to the routine use of Diclofenac and in 1000 patients receiving 100 mg Diclofenac before ERCP. RESULTS: PEP occurred in 56 (2.8%) of the 2000 patients, and the rate of the pancreatitis was 2.8% in control group and 2.8% in Diclofenac group (p = 1.000). The PEP rate among the native papilla patients was 3.9% in control group and 3.6% in Diclofenac group (p = 0.803). In subgroup analysis of patients with a high risk of PEP, Diclofenac neither prevented PEP nor made its course milder. CONCLUSIONS: In an unselected patient population in a center with a low incidence of PEP, Diclofenac seems to have no beneficial effect.
Diclofenac-Based Hydrazones and Spirothiazolidinones: Synthesis, Characterization, and Antimicrobial Properties.[Pubmed:28370198]
Arch Pharm (Weinheim). 2017 May;350(5).
We report here the synthesis, structural characterization, and biological evaluation of novel Diclofenac-based hydrazone (4a-f) and spirothiazolidinone (5a-f, 6a-f) derivatives designed as potential antimicrobial agents. The compounds were evaluated in vitro for their antiviral activity against a wide spectrum of DNA and RNA viruses. They were further screened in vitro against different strains of bacteria and fungi. The hydrazone derivatives, 4a and 4c-f, were found to be active against herpesviruses (HSV-1, HSV-2, and HSV-1 TK(-) ), vaccinia virus, and Coxsackie B4 virus, with EC50 values between 6.6 microg/mL and 14.7 mug/mL, and the selectivity index values were greater than 10 for 4a and 4f. The newly synthesized compounds (4-6) were inactive against the bacterial and the fungal strains tested, at levels below 2500, 1250, or 625 mug/mL. Interestingly, the key intermediate 3 with a free hydrazide moiety displayed antifungal properties against Candida albicans and C. parapsilosis at MIC values of 4.88 microg/mL and 78.12 mug/mL, respectively.
Analgesic Effect of Topical Sodium Diclofenac before Retinal Photocoagulation for Diabetic Retinopathy: A Randomized Double-masked Placebo-controlled Intraindividual Crossover Clinical Trial.[Pubmed:28367037]
Korean J Ophthalmol. 2017 Apr;31(2):102-107.
PURPOSE: To evaluate the analgesic effect of topical sodium Diclofenac 0.1% before retinal laser photocoagulation for diabetic retinopathy. METHODS: Diabetic patients who were candidates for peripheral laser photocoagulation were included in a randomized, placebo-controlled, intraindividual, two-period, and crossover clinical trial. At the first session and based on randomization, one eye received topical sodium Diclofenac 0.1% and the other eye received an artificial tear drop (as placebo) three times before laser treatment. At the second session, eyes were given the alternate drug. Patients scored their pain using visual analogue scale (max, 10 cm) at both sessions. Patients and the surgeon were blinded to the drops given. Difference of pain level was the main outcome measure. RESULTS: A total of 200 eyes of 100 patients were enrolled. Both treatments were matched regarding the applied laser. Pain sensation based on visual analogue scale was 5.6 +/- 3.0 in the treated group and 5.5 +/- 3.0 in the control group. The calculated treatment effect was 0.15 (95% confidence interval, -0.27 to 0.58; p = 0.486). The estimated period effect was 0.24 (p = 0.530) and the carryover effect was not significant (p = 0.283). CONCLUSIONS: Pretreatment with topical sodium Diclofenac 0.1% does not have any analgesic effect during peripheral retinal laser photocoagulation in diabetic patients.
In vitro drug interaction of levocetirizine and diclofenac: Theoretical and spectroscopic studies.[Pubmed:28371723]
Spectrochim Acta A Mol Biomol Spectrosc. 2017 Jun 15;181:239-248.
Levocetirizine dihydrochloride is known to interact with some anti-inflammatory drugs. We report here a comprehensive integrated theoretical and experimental study for the in vitro drug interaction between levocetirizine dihydrochloride (LEV) and Diclofenac sodium (DIC). The interaction of the two drugs was confirmed by the molecular ion peak obtained from the mass spectrum of the product. Moreover, FTIR and (1)HNMR spectra of the individual drugs and their interaction product were inspected to allocate the possible sites of interaction. In addition, quantum mechanical DFT calculations were performed to search for the interaction sites and to verify the types of interactions deduced from the spectroscopic studies such as charge-transfer and non-bonding pi-pi interactions. It was found that the studied drugs interact with each other in aqueous solution via four types of interactions, namely, ion-pair formation, three weak hydrogen bonds, non-bonding pi-pi interactions and charge-transfer from DIC to LEV.


