DehydrocurdioneCAS# 38230-32-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 38230-32-9 | SDF | Download SDF |
| PubChem ID | 14191392 | Appearance | Powder |
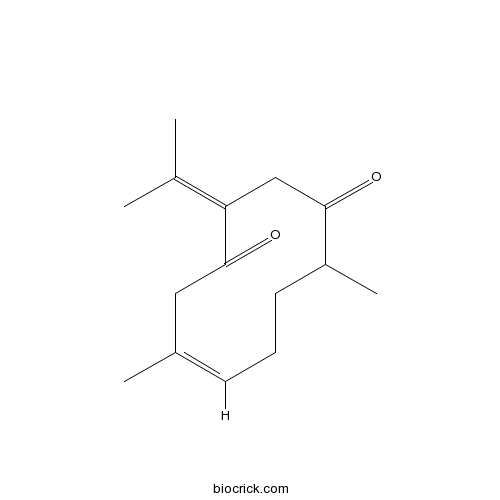
| Formula | C15H22O2 | M.Wt | 234.33 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (6Z)-6,10-dimethyl-3-propan-2-ylidenecyclodec-6-ene-1,4-dione | ||
| SMILES | CC1CCC=C(CC(=O)C(=C(C)C)CC1=O)C | ||
| Standard InChIKey | ZYPUZCWWTYIGFV-WDZFZDKYSA-N | ||
| Standard InChI | InChI=1S/C15H22O2/c1-10(2)13-9-14(16)12(4)7-5-6-11(3)8-15(13)17/h6,12H,5,7-9H2,1-4H3/b11-6- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dehydrocurdione Dilution Calculator

Dehydrocurdione Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2675 mL | 21.3374 mL | 42.6749 mL | 85.3497 mL | 106.6872 mL |
| 5 mM | 0.8535 mL | 4.2675 mL | 8.535 mL | 17.0699 mL | 21.3374 mL |
| 10 mM | 0.4267 mL | 2.1337 mL | 4.2675 mL | 8.535 mL | 10.6687 mL |
| 50 mM | 0.0853 mL | 0.4267 mL | 0.8535 mL | 1.707 mL | 2.1337 mL |
| 100 mM | 0.0427 mL | 0.2134 mL | 0.4267 mL | 0.8535 mL | 1.0669 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kaempferol 3,7-di-O-glucoside
Catalog No.:BCN9114
CAS No.:25615-14-9
- Kaempferol 3-O-rutinoside 7-O-glucoside
Catalog No.:BCN9113
CAS No.:34336-18-0
- Kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside
Catalog No.:BCN9112
CAS No.:476617-49-9
- Plantanone B
Catalog No.:BCN9111
CAS No.:55780-30-8
- Rebaudioside N
Catalog No.:BCN9110
CAS No.:1220616-46-5
- Epigambogic acid
Catalog No.:BCN9109
CAS No.:887606-04-4
- Bornyl Acetate
Catalog No.:BCN9108
CAS No.:76-49-3
- Loureirin D
Catalog No.:BCN9107
CAS No.:119425-91-1
- (±)-Vasicine
Catalog No.:BCN9106
CAS No.:6159-56-4
- Macrozamin
Catalog No.:BCN9105
CAS No.:6327-93-1
- 4H-1-Benzopyran-4-one, 2,3-dihydro-3,5,7-trihydroxy-3-[(4-methoxyphenyl)methyl]-, (R)-
Catalog No.:BCN9104
CAS No.:118204-64-1
- (3R)-2,3-Dihydro-5,7-dihydroxy-3-[(4-hydroxyphenyl)methyl]-4H-1-benzopyran-4-one
Catalog No.:BCN9103
CAS No.:849727-88-4
- Isoverticine
Catalog No.:BCN9116
CAS No.:23496-43-7
- Bancroftinone
Catalog No.:BCN9117
CAS No.:14964-98-8
- 5-O-Cinnamoylquinic acid
Catalog No.:BCN9118
CAS No.:6470-68-4
- Valeriandoid F
Catalog No.:BCN9119
CAS No.:1427162-60-4
- Bletilloside A
Catalog No.:BCN9120
CAS No.:2292159-89-6
- Rapanone
Catalog No.:BCN9121
CAS No.:573-40-0
- Bacopaside IV
Catalog No.:BCN9122
CAS No.:155545-03-2
- Itaconic acid
Catalog No.:BCN9123
CAS No.:97-65-4
- Arundinin
Catalog No.:BCN9124
CAS No.:148225-38-1
- 3'-O-Methylbatatasin III
Catalog No.:BCN9125
CAS No.:101330-69-2
- Bacopaside N1
Catalog No.:BCN9126
CAS No.:871706-74-0
- γ-Asarone
Catalog No.:BCN9127
CAS No.:5353-15-1
Bioactive chemical constituents from Curcuma caesia Roxb. rhizomes and inhibitory effect of curcuzederone on the migration of triple-negative breast cancer cell line MDA-MB-231.[Pubmed:31726856]
Nat Prod Res. 2019 Nov 15:1-5.
Rhizomes of Curcuma caesia are traditionally used to treat cancer in India. The aim is to isolate chemical constituents from C. caesia rhizomes through bioassay-guided fractionation. The extract, hexanes and chloroform fractions showed effect on MCF-7 and MDA-MB-231cells in cell viability assay. The chromatographic separation afforded germacrone (1), zerumbone (2), furanodienone (3), curzerenone (4), curcumenol (5), zederone (6), curcumenone (7), Dehydrocurdione (8) from hexanes fraction and curcuminol G (9), curcuzederone (10), (1S, 10S), (4S,5S)-germacrone-1 (10), 4-diepoxide (11), wenyujinin B (12), alismoxide (13), aerugidiol (14), zedoarolide B (15), zedoalactone B (16), zedoarondiol (17), isozedoarondiol (18) from chloroform fraction. This is first report of compounds 2, 9-13, 15-18 from C. caesia. The study demonstrated compounds 1-4 and 10 are the bioactive compounds. The effect of curcuzederone (10) on MDA-MB-231 cell migration showed significant inhibition in scratch and Transwell migration assays. The results revealed that curcuzederone could be a promising drug to treat cancer.
Curcuma sp.-derived dehydrocurdione induces heme oxygenase-1 through a Michael reaction between its alpha, beta-unsaturated carbonyl and Keap1.[Pubmed:29356228]
Phytother Res. 2018 May;32(5):892-897.
To elucidate the anti-inflammatory mechanism of Curcuma sp., we investigated whether Dehydrocurdione, a sesquiterpene contained in Curcuma sp., induces heme oxygenase (HO)-1, an antioxidative enzyme, in RAW 264.7 macrophages. Dehydrocurdione was extracted from the rhizome of Curcuma sp., and its purity was verified by high performance liquid chromatography. Treatment with 10-100 muM Dehydrocurdione transiently and concentration-dependently increased HO-1 mRNA and protein levels. Docking simulation suggested the presence of the Michael reaction between Dehydrocurdione and Kelch-like ECH-associated protein (Keap)1 keeping nuclear factor-erythroid2-related-factor (Nrf)2, a transcription factor, in the cytoplasm. Nrf2 that was definitely free from Keap1 was detected in the nuclei after Dehydrocurdione treatment. Subsequently, the HO-1 E2 enhancer, a target of Nrf2, was activated, resulting in HO-1 expression. Also, an investigation using 6-shogaol and 6-gingerol supported the concept that the alpha, beta-unsaturated carbonyl structure plays an important role in the interaction with Keap1. Dehydrocurdione suppressed lipopolysaccharide-induced NO release, a marker of inflammation. Clarification of the HO-1 synthesis increase mechanism revealed in this study will help contribute to the development of novel phytotherapeutic strategies against inflammation-associated diseases.
Cytotoxicity and inhibition of leukemic cell proliferation by sesquiterpenes from rhizomes of Mah-Lueang (Curcuma cf. viridiflora Roxb.).[Pubmed:29274817]
Bioorg Med Chem Lett. 2018 Feb 1;28(3):410-414.
Curcuma cf. viridiflora Roxb., also known as Mah-Lueang in Thai, belongs to the Zingiberaceae family and is grown from rhizomes. The rhizome of the plant has been used for medicinal purposes, in particular, to treat paralysis in Thai traditional medicine. However, no biologically active compounds have been reported from Mah-Lueang yet. In this study, natural compounds were isolated from Mah-Lueang and structurally determined by spectroscopic methods, including electrospray ionization mass spectrometry and nuclear magnetic resonance. The four isolated compounds were identified as furanodiene (1), Dehydrocurdione (2), germacrone-4,5-epoxide (3), and zedoarondiol (4). These sesquiterpenes were investigated for antileukemic activities against KG1a and Molt4 cells. Leukemic cell proliferation is regulated by the Wilms' tumor 1 (WT1) transcription factor. Compound 1 showed the strongest cytotoxicity against both KG1a and Molt4 cells. Noncytotoxic concentrations (20% inhibitory concentration values) of all compounds were able to decrease the WT1 protein expression and total cell numbers in both cell lines. The four compounds showed good inhibitory activities for WT1 protein expression. Compounds 3 and 4 showed excellent antileukemic activities for both cell lines. In summary, four sesquiterpene compounds with antileukemic activities against the KG1a and Molt4 cell lines were identified in Mah-Lueang extracts.
Cytotoxic constituents from the rhizomes of Curcuma zedoaria.[Pubmed:25126594]
ScientificWorldJournal. 2014;2014:321943.
Curcuma zedoaria also known as Temu putih is traditionally used in food preparations and treatment of various ailments including cancer. The cytotoxic activity of hexane, dichloromethane, ethyl acetate, methanol, and the methanol-soxhlet extracts of Curcuma zedoaria rhizomes was tested on two human cancer cell lines (Ca Ski and MCF-7) and a noncancer cell line (HUVEC) using MTT assay. Investigation on the chemical components in the hexane and dichloromethane fractions gave 19 compounds, namely, labda-8(17),12 diene-15,16 dial (1), Dehydrocurdione (2), curcumenone (3), comosone II (4), curcumenol (5), procurcumenol (6), germacrone (7), zerumbone epoxide (8), zederone (9), 9-isopropylidene-2,6-dimethyl-11-oxatricyclo[6.2.1.0(1,5)]undec-6-en-8-ol (10), furanodiene (11), germacrone-4,5-epoxide (12), calcaratarin A (13), isoprocurcumenol (14), germacrone-1,10-epoxide (15), zerumin A (16), curcumanolide A (17), curcuzedoalide (18), and gweicurculactone (19). Compounds (1-19) were evaluated for their antiproliferative effect using MTT assay against four cancer cell lines (Ca Ski, MCF-7, PC-3, and HT-29). Curcumenone (3) and curcumenol (5) displayed strong antiproliferative activity (IC50 = 8.3 +/- 1.0 and 9.3 +/- 0.3 mug/mL, resp.) and were found to induce apoptotic cell death on MCF-7 cells using phase contrast and Hoechst 33342/PI double-staining assay. Thus, the present study provides basis for the ethnomedical application of Curcuma zedoaria in the treatment of breast cancer.
Quantitative analysis and discrimination of steamed and non-steamed rhizomes of Curcuma wenyujin by GC-MS and HPLC.[Pubmed:24105919]
J Chromatogr Sci. 2014 Oct;52(9):961-70.
Simple gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC) methods were developed for quantifying eight volatile compounds and 10 sesquiterpenoids, respectively. GC-MS analysis was performed on an HP-5MS capillary column (30 mx0.25 mm i.d.) coated with 0.25 mum film 5% phenyl-95% methylpolysiloxane and selected ion monitoring was used for quantification. Four volatile and previously unquantified monoterpenoids were determined. HPLC analysis was performed on a C18 column with water and acetonitrile as mobile phase. The proposed method, determined 10 non-polar and polar sesquiterpenoids simultaneously, which covered a wider polar range of analytes and had a more perfect resolution. Among them, five sesquiterpenoids were not determined before and some specific components, (4S,5S)-germacrone-4,5-epoxide, curcumenone and Dehydrocurdione were completely separated for the first time. Both methods were validated for linearity, limit of detection and quantification, precision, accuracy, recovery and system suitability. The methods were simple, effective, reliable and successfully applied to global detection and analysis of volatile and non-volatile components of steamed and non-steamed rhizomes of Curcuma wenyujin (Wen-E-Zhu (WEZ) and Pian-Jiang-Huang (PJH)). Multivariate statistical analysis was employed to distinguish PJH and WEZ and seven chemical components, including (4S,5S)-germacrone-4,5-epoxide, curcumenone, beta-elemene, curzerene, borneol, isoborneol and camphor, were screened as chemical markers. The present study provided a promising method for accurate discrimination of the herbal medicines with the same origin.
Anti-androgenic effect of sesquiterpenes isolated from the rhizomes of Curcuma aeruginosa Roxb.[Pubmed:22465508]
Fitoterapia. 2012 Jul;83(5):864-71.
Six sesquiterpenes: germacrone (1), zederone (2), Dehydrocurdione (3), curcumenol (4), zedoarondiol (5) and isocurcumenol (6) were isolated from rhizomes of Curcuma aeruginosa Roxb. (Zingiberaceae). They inhibited 5alpha-reductase which converts testosterone to dihydrotestosterone (DHT). Germacrone (1) was the most potent (IC(50)=0.42+/-0.05 mg/mL). Compound 1 was anti-androgenic in LNCaP cells when proliferation was testosterone-induced. The growth of flank gland of male Syrian hamsters is dependent on circulating androgen and when maintained with testosterone, 1 (3, 30, 100mug) inhibited growth but was ineffective against DHT. The similar activity profile was observed on the 5alpha-reductase inhibitor, finasteride (100 mug) treatment group. The androgen receptor binding assay showed that 1 did not bind to the androgen receptor. In conclusion, 1 showed anti-androgenic effect on in vitro and in vivo assays. One of the possible mechanisms was inhibition 5alpha-reductase activity. Thus, 1 is a potential lead compound for treatment of androgen-dependent disorders.
Sesquiterpenes from Curcuma comosa.[Pubmed:18663560]
J Nat Med. 2009 Jan;63(1):102-4.
From the dried rhizomes of Curcuma comosa cultivating in Thailand, 26 known sesquiterpenes were isolated: zederone, zederone epoxide, furanodienone, isofuranodienone, 1(10)Z,4Z-furanodiene-6-one, glechomanolide, Dehydrocurdione, neocurdione, curdione, 7 alpha-hydroxyneocurdione, 7 beta-hydroxycurdione, germacrone-1(10),4-diepoxide, germacrone, 13-hydroxygermacrone, curzerenone, curcolonol, alismol, alismoxide, zedoarondiol, isozedoarondiol, procurcumenol, isoprocurcumenol, aerugidiol, zedoalactone B, curcumenone, and curcumadione. Their structures were elucidated on the basis of physicochemical evidence. Among them, glechomanolide, curzerenone, curcolonol, alismol, alismoxide, and zedoarondiol showed no significant optical activities, so they may be artifact products during the isolation or drying process.
Anti-inflammatory sesquiterpenes from Curcuma zedoaria.[Pubmed:16901812]
Nat Prod Res. 2006 Jun;20(7):680-5.
From the methanolic extract of the rhizome of Curcuma zedoaria, we isolated anti-inflammatory sesquiterpene furanodiene (1) and furanodienone (2) along with new sesquiterpene compound 3 and known eight sesquiterpenes, zederone (4), curzerenone (5), curzeone (6), germacrone (7), 13-hydroxygermacrone (8), Dehydrocurdione (9), curcumenone (10), and zedoaronediol (11). Their structures were elucidated on the basis of spectroscopic data. The anti-inflammatory effect of isolated components on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation of mouse ears were examined. Compounds 1 and 2 suppressed the TPA-induced inflammation of mouse ears by 75% and 53%, respectively, at a dose of 1.0 micromol. Their activities are comparable to that of indomethacin, the normally used anti-inflammatory agent.
Zedoariae rhizoma and curcumin inhibits platelet-derived growth factor-induced proliferation of human hepatic myofibroblasts.[Pubmed:15683851]
Int Immunopharmacol. 2005 Mar;5(3):555-69.
During the course of liver fibrogenesis, hepatic myofibroblast cells (hMF), mostly derived from hepatic stellate cells (HSC), proliferate and synthesize excessive amounts of extracellular matrix (ECM) components. To evaluate the antiproliferative effect of a traditional herbal medicine, Zedoariae rhizoma water extracts (ZR) was examined on the growth inhibition of human hMF since proliferation of hMF is known to be central for the development of fibrosis during liver injury, and factors that may limit their growth are potential antifibrotic agents. The aim of this study was to test the effects of ZR on the proliferation and to clarify the molecular mechanisms of ZR inhibition of HSC proliferation in cultured human hMF. The cells were stimulated by platelet-derived growth factor (PDGF)-BB in the presence or absence of ZR. Proliferation was determined by bromodeoxy-uridine (BrdU) incorporation. The mRNA expressions of collagen alpha1(I) and (IV) were evaluated by a quantitative reverse transcription-polymerase chain reaction (RT-PCR). PDGF-receptor tyrosine phosphorylation was detected using anti-phosphotyrosine antibody. PDGF-receptor radioligand binding assay was performed by [125I]PDGF-BB. ZR inhibited the PDGF-BB-induced cell-proliferation and collagen alpha1(I) and (IV) mRNA expressions. ZR reduced the autophosphorylation of the PDGF-receptor. ZR blocked PDGF-BB binding to its receptor in a non-competitive manner. Furthermore, the 80% aqueous acetone extract of ZR was also found to show a decreasing effect against the proportion of S phase cells after PDGF stimulation. To clarify the active compounds, the principal constituents of seven sesquiterpenes (curdione, Dehydrocurdione, germacrone, curcumenol, isocurcumenol, zedoarondiol and curcumenone) and a diarylheptanoid (curcumin) were examined. Among them, curcumin was found to decrease the proportion of S phase cells after PDGF stimulation at a dose of 30-50 microM. Potent antiproliferative and antifibrogenic effects of ZR toward hMF indicated that ZR might have therapeutic implications in chronic liver disease, indicating a novel role for ZR as a growth inhibitory mediator and pointing out its potential involvement in the negative regulation of liver fibrogenesis. In conclusion, ZR has an inhibitory effect on PDGF-induced proliferation of hMF and the blocking of PDGF-BB binding to its receptor may be the mechanism behind this effect.
Medicinal foodstuffs. XXIX. Potent protective effects of sesquiterpenes and curcumin from Zedoariae Rhizoma on liver injury induced by D-galactosamine/lipopolysaccharide or tumor necrosis factor-alpha.[Pubmed:12033504]
Biol Pharm Bull. 2002 May;25(5):627-31.
The 80% aqueous acetone extract of Zedoariae Rhizoma was found to show a protective effect against D-galactosamine (D-GalN)/lipopolysaccharide-induced acute liver injury in mice. To clarify the active compounds, the principal constituents were examined and 11 sesquiterpenes (furanodiene, curdione, neocurdrione, Dehydrocurdione, germacrone, 13-hydroxygermacrone, curcumenol, isocurcumenol, aerugidiol, zedoarondiol, and curcumenone) and a diarylheptanoid (curcumin) were found to inhibit the increase in serum aspartate aminotransaminase and alanine aminotransaminase at a dose of 50 mg/kg p.o. in agreement with the previous in vitro studies, except for Dehydrocurdione, aerugidiol, and zedoarondiol. In particular, curdione, neocurdione, curcumenol, and isocurcumenol potently inhibited the increase at a dose of 12.5 mg/kg p.o. Furthermore, the eight sesquiterpenes, furanodiene, curdione, neocurdione, Dehydrocurdione, germacrone, 13-hydroxygermacrone, curcumenol, and curcumenone, also showed a protective effect against D-GalN/tumor necrosis factor-alpha-induced liver injury in mice at a dose of 50 mg/kg p.o.
A Ca(2+) channel blocker-like effect of dehydrocurdione on rodent intestinal and vascular smooth muscle.[Pubmed:10973625]
Eur J Pharmacol. 2000 Sep 8;403(3):235-42.
Effects of Dehydrocurdione, a zedoary-derived sesquiterpene, on smooth muscle were investigated by recording the mechanical activity of intestines and aorta from guinea pigs and rats. Dehydrocurdione (0.1-3 mM) induced a sustained relaxation of rat duodenum and inhibited spontaneous motility. Dehydrocurdione (0.1-1 mM) inhibited the contractile response of guinea pig ileum induced by acetylcholine (0.01-10 microM), histamine (0.03-10 microM) and substance P (0.1-30 nM) in a non-competitive manner. Acetylcholine (0.5 microM) elicited a transient contraction followed by a sustained contraction of guinea pig ileum, and Dehydrocurdione pretreatment inhibited the sustained component, which depends on Ca(2+) entry from the extracellular space. The high K(+)-induced contraction of rat aortic ring is reported to be blocked by Ca(2+) channel blockers, while the norepinephrine-induced contraction includes a Ca(2+) channel blocker-resistant component. Dehydrocurdione (1 mM) blocked the high K(+) (60 mM)-induced contraction of rat aortic ring by 81%, while it inhibited the norepinephrine (1 microM)-induced contraction by only 28%. Dehydrocurdione (1 mM) significantly reduced the high K(+)-stimulated increase in cytosolic Ca(2+) level of Fura-2-loaded mesenteric artery from rats. These results suggest that the inhibitory effects of Dehydrocurdione on intestinal and vascular smooth muscle are mediated by blockade of Ca(2+) entry from the extracellular space.
Antiinflammatory potency of dehydrocurdione, a zedoary-derived sesquiterpene.[Pubmed:9892041]
Inflamm Res. 1998 Dec;47(12):476-81.
OBJECTIVE AND DESIGN: Dehydrocurdione, a sesquiterpene isolated from zedoary, was tested for in vivo and in vitro antiinflammatory actions. MATERIALS: Analgesic effect was tested in ICR mice by the acetic acid-induced writhing method. Antipyretic effect was studied in Sprague-Dawley rats treated with baker's yeast. Antiinflammatory activities were tested in Wistar rats with carrageenan-induced paw edema and adjuvant-induced chronic arthritis. In vitro analyses included the capabilities to inhibit cyclooxygenase activity, and to scavenge free radicals as determined by electron paramagnetic resonance (EPR). RESULTS: Oral administration of Dehydrocurdione (40 to 200 mg/kg) mitigated the writhing reflex. induced by acetic acid and the fever elicited by baker's yeast. A higher dose (200 mg/kg) of Dehydrocurdione was required to inhibit the carrageenan-induced paw edema. Oral administration of Dehydrocurdione at 120 mg/kg/day for 12 days significantly reduced chronic adjuvant arthritis. Unlike indomethacin (IC50: 0.1 microM), Dehydrocurdione showed minimal cyclooxygenase inhibition. However, Dehydrocurdione (100 microM to 5 mM) significantly reduced free radical formation from hydrogen peroxide and ferrous iron determined by EPR spectrometry using 5,5'-dimethyl-1-pyrroline-N-oxide as a spin trap agent. CONCLUSION: In addition to the well-known effect of zedoary as a stomachic, Dehydrocurdione, the major component of Curcuma zedoaria Roscoe has antiinflammatory potency related to its antioxidant effect.


