DehydrocrebanineCAS# 77784-22-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 77784-22-6 | SDF | Download SDF |
| PubChem ID | 149600 | Appearance | Yellow powder |
| Formula | C20H19NO4 | M.Wt | 337.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
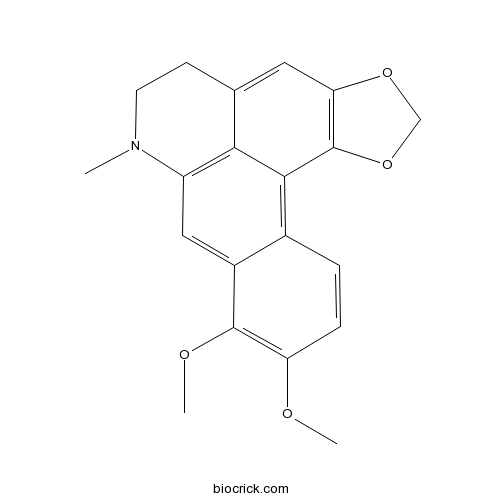
| SMILES | CN1CCC2=CC3=C(C4=C5C=CC(=C(C5=CC1=C24)OC)OC)OCO3 | ||
| Standard InChIKey | HCKFFNXOLCSPAI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H19NO4/c1-21-7-6-11-8-16-20(25-10-24-16)18-12-4-5-15(22-2)19(23-3)13(12)9-14(21)17(11)18/h4-5,8-9H,6-7,10H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dehydrocrebanine has strong activity against promyelocytic leukemia cells (HL-60) with an IC50 of 2.14 ug/mL. 2. Dehydrocrebanine shows potent antimalarial activity with an IC50 value of 70 ng/ml. |
| Targets | Antifection |

Dehydrocrebanine Dilution Calculator

Dehydrocrebanine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9638 mL | 14.8192 mL | 29.6384 mL | 59.2768 mL | 74.096 mL |
| 5 mM | 0.5928 mL | 2.9638 mL | 5.9277 mL | 11.8554 mL | 14.8192 mL |
| 10 mM | 0.2964 mL | 1.4819 mL | 2.9638 mL | 5.9277 mL | 7.4096 mL |
| 50 mM | 0.0593 mL | 0.2964 mL | 0.5928 mL | 1.1855 mL | 1.4819 mL |
| 100 mM | 0.0296 mL | 0.1482 mL | 0.2964 mL | 0.5928 mL | 0.741 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Potassium phosphate monobasic
Catalog No.:BCC7583
CAS No.:7778-77-0
- 1,2-Dihydrotanshinquinone
Catalog No.:BCN2477
CAS No.:77769-21-2
- 1,7-Dihydroxy-3-methoxy-2-prenylxanthone
Catalog No.:BCN1354
CAS No.:77741-58-3
- (-)-Toddanol
Catalog No.:BCN3429
CAS No.:77715-99-2
- Arctigenin
Catalog No.:BCN6291
CAS No.:7770-78-7
- 6'''-Feruloylspinosin
Catalog No.:BCN2802
CAS No.:77690-92-7
- Enoximone
Catalog No.:BCC7155
CAS No.:77671-31-9
- ent-11,16-Epoxy-15-hydroxykauran-19-oic acid
Catalog No.:BCN1355
CAS No.:77658-46-9
- ent-9-Hydroxy-15-oxo-19-kauranoic acid
Catalog No.:BCN1356
CAS No.:77658-45-8
- ent-9-Hydroxy-15-oxo-16-kauren-19-oic acid
Catalog No.:BCN1357
CAS No.:77658-39-0
- Pterokaurene L3
Catalog No.:BCN4583
CAS No.:77658-38-9
- Boc-D-Ala-OH
Catalog No.:BCC3049
CAS No.:7764-95-6
- Carasinol B
Catalog No.:BCN8226
CAS No.:777857-86-0
- GNF 2
Catalog No.:BCC3891
CAS No.:778270-11-4
- GNF 5
Catalog No.:BCC3892
CAS No.:778277-15-9
- Nelumol A
Catalog No.:BCN4749
CAS No.:77836-86-3
- (1R)-(+)-Alpha-Pinene
Catalog No.:BCC8275
CAS No.:7785-70-8
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- Isopulegol
Catalog No.:BCN4974
CAS No.:7786-67-6
- Beta-Lipotropin (1-10), porcine
Catalog No.:BCC1009
CAS No.:77875-68-4
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- Longikaurin E
Catalog No.:BCN4329
CAS No.:77949-42-9
- Z-Glycinol
Catalog No.:BCC3095
CAS No.:77987-49-6
Antimalarials from Stephania venosa, Prismatomeris sessiliflora, Diospyros montana and Murraya siamensis.[Pubmed:10630122]
Planta Med. 1999 Dec;65(8):754-6.
Fourteen compounds isolated from Stephania venosa, Prismatomeris sessiliflora, Diospyros montana and Murraya siamensis were tested for their antimalarial potential. The 6a,7-dehydroaporphine alkaloids dehydrostephanine and Dehydrocrebanine showed potent activity with IC50 values of 40 and 70 ng/ml, respectively. The 13C-NMR data of rubiadin, rubiadin-1-methyl ether, diospyrin and 5-hydroxy-4-methoxy-2-naphthal-dehyde were extensively studied.
Cytotoxic and antimicrobial activities of aporphine alkaloids isolated from Stephania venosa (Blume) Spreng.[Pubmed:21305448]
Planta Med. 2011 Sep;77(13):1519-24.
The cytotoxic activity of five alkaloids, namely 4,5-dioxo-Dehydrocrebanine (1), Dehydrocrebanine (2), crebanine (3), oxostephanine (4), and thailandine (5) isolated from the tuber and leaves of Stephania venosa (Blume) Spreng was investigated. Thailandine showed the strongest activity against lung carcinoma cells (A549) (IC50 of 0.30 microg/mL) with very low cytotoxicity against normal embryonic lung cells (MRC-5). Thailandine also demonstrated strong activity against Plasmodium falciparum, K1 strain (IC50 of 20 ng/mL), and Mycobacterium tuberculosis H(37)Ra (MIC of 6.25 microg/mL) as well as gram-positive bacteria such as Streptococcus pneumoniae and Staphylococcus aureus. Oxostephanine exhibited strong activity against breast cancer (BC) and acute lymphoblastic leukemia cells (MOLT-3) with an IC50 of 0.24 and 0.71 microg/mL, respectively, and exhibited very low cytotoxicity against MRC-5 cells. Dehydrocrebanine demonstrated strong activity against promyelocytic leukemia cells (HL-60) with an IC50 of 2.14 microg/mL whereas crebanine showed weak activity against cancer cell lines. However, both of them showed cytotoxicity against MRC-5 cells.
The first total syntheses of (+/-)-norphoebine, dehydrophoebine, oxophoebine, dehydrocrebanine, oxocrebanine and uthongine and their cytotoxicity against three human cancer cell lines.[Pubmed:27146697]
J Asian Nat Prod Res. 2016 Nov;18(11):1042-56.
The first total syntheses of (+/-)-norphoebine, dehydrophoebine, oxophoebine, Dehydrocrebanine, oxocrebanine and uthongine have been achieved. The crucial step involved the formation of ring C by a microwave-assisted direct biaryl coupling to produce the aporphine skeleton in high yields. The synthetic alkaloids were evaluated for their cytotoxicity against three human cancer cell lines MCF7, KB and NCI-H187. The results showed that uthongine was the best candidate of the series and it exhibited cytotoxicity against a human breast cancer MCF7 line with an IC50 = 3.05 muM.


