D-Tartaric acidCAS# 147-71-7 |
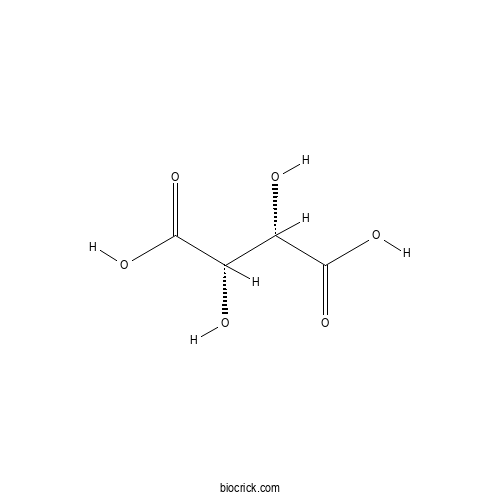
- D(-)-Tartaric acid
Catalog No.:BCN8460
CAS No.:526-83-0
- Tartaric acid
Catalog No.:BCN3824
CAS No.:87-69-4
- DL-Tartaric acid
Catalog No.:BCN9052
CAS No.:133-37-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 147-71-7 | SDF | Download SDF |
| PubChem ID | 439655.0 | Appearance | Powder |
| Formula | C4H6O6 | M.Wt | 150.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Unnatural tartaric acid | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3S)-2,3-dihydroxybutanedioic acid | ||
| SMILES | C(C(C(=O)O)O)(C(=O)O)O | ||
| Standard InChIKey | FEWJPZIEWOKRBE-LWMBPPNESA-N | ||
| Standard InChI | InChI=1S/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10)/t1-,2-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

D-Tartaric acid Dilution Calculator

D-Tartaric acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.6627 mL | 33.3133 mL | 66.6267 mL | 133.2534 mL | 166.5667 mL |
| 5 mM | 1.3325 mL | 6.6627 mL | 13.3253 mL | 26.6507 mL | 33.3133 mL |
| 10 mM | 0.6663 mL | 3.3313 mL | 6.6627 mL | 13.3253 mL | 16.6567 mL |
| 50 mM | 0.1333 mL | 0.6663 mL | 1.3325 mL | 2.6651 mL | 3.3313 mL |
| 100 mM | 0.0666 mL | 0.3331 mL | 0.6663 mL | 1.3325 mL | 1.6657 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Coniferylaldehydel
Catalog No.:BCX0648
CAS No.:458-36-6
- Genistein 8-C-glucoside
Catalog No.:BCX0647
CAS No.:66026-80-0
- Phytosphingosine
Catalog No.:BCX0646
CAS No.:554-62-1
- Apigenin-6-C-β-D-xylopyranosyl-8-C-α-L-arabinopyranoside
Catalog No.:BCX0645
CAS No.:85700-46-5
- L-xylose
Catalog No.:BCX0644
CAS No.:609-06-3
- 8-Epi-Loganic acid-6'-O-β-D-glucoside
Catalog No.:BCX0643
CAS No.:176226-39-4
- Isokadsuranin
Catalog No.:BCX0642
CAS No.:82467-52-5
- 2-O-β-D-Glucopyranosyl-L-ascorbic acid
Catalog No.:BCX0641
CAS No.:562043-82-7
- Gymnoside IX
Catalog No.:BCX0640
CAS No.:898827-00-4
- Palmitoleic acid methyl ester
Catalog No.:BCX0639
CAS No.:1120-25-8
- Schiarisanrin A
Catalog No.:BCX0638
CAS No.:130252-41-4
- Lucidin Omega-Methyl Ether
Catalog No.:BCX0637
CAS No.:79560-36-4
- 4-Ethoxybenzyl alcohol
Catalog No.:BCX0650
CAS No.:6214-44-4
- Dehydrosulphurenic acid
Catalog No.:BCX0651
CAS No.:175615-56-2
- Coumalic acid
Catalog No.:BCX0652
CAS No.:500-05-0
- 3-Indoleacetamide
Catalog No.:BCX0653
CAS No.:879-37-8
- 8,9-epoxy-3-isobutyryloxy-10-(2-methylbutanoyl)thymol
Catalog No.:BCX0654
CAS No.:22518-07-6
- 4-O-galloylalbiflorin
Catalog No.:BCX0655
CAS No.:1201580-97-3
- Glycyroside
Catalog No.:BCX0656
CAS No.:125310-04-5
- Acid Red 73
Catalog No.:BCX0657
CAS No.:5413-75-2
- 3-O-Coumaroylquinic acid
Catalog No.:BCX0658
CAS No.:87099-71-6
- 6'-O-galloylalbiflorin
Catalog No.:BCX0659
CAS No.:929042-36-4
- Filbertone
Catalog No.:BCX0660
CAS No.:81925-81-7
- Azorubin
Catalog No.:BCX0661
CAS No.:3567-69-9
From desert flora to cancer therapy: systematic exploration of multi-pathway mechanisms using network pharmacology and molecular modeling approaches.[Pubmed:38666020]
Front Pharmacol. 2024 Apr 11;15:1345415.
Ovarian cancer, often labeled a "silent killer," remains one of the most compelling and challenging areas of cancer research. In 2019 alone, a staggering 222,240 new cases of ovarian cancer were reported, with nearly 14,170 lives tragically lost to this relentless disease. The absence of effective diagnostic methods, increased resistance to chemotherapy, and the heterogeneous nature of ovarian cancer collectively contribute to the unfavorable prognosis observed in the majority of cases. Thus, there is a pressing need to explore therapeutic interventions that offer superior efficacy and safety, thereby enhancing the survival prospects for ovarian cancer patients. Recognizing this potential, our research synergizes bioinformatics with a network pharmacology approach to investigate the underlying molecular interactions of Saudi Arabian flora (Onopordum heteracanthum, Acacia ehrenbergiana, Osteospermum vaillantii, Cyperus rotundus, Carissa carandas, Carissa spinarum, and Camellia sinensis) in ovarian cancer treatment. At first, phytoconstituents of indigenous flora and their associated gene targets, particularly those pertinent to ovarian cancer, were obtained from open-access databases. Later, the shared targets of plants and diseases were compared to identify common targets. A protein-protein interaction (PPI) network of predicted targets was then constructed for the identification of key genes having the highest degree of connectivity among networks. Following that, a compound-target protein-pathway network was constructed, which uncovered that, namely, hispidulin, stigmasterol, ascorbic acid, octopamine, cyperene, kaempferol, pungenin, citric acid, D-Tartaric acid, beta-sitosterol, (-)-epicatechin gallate, and (+)-catechin demonstrably influence cell proliferation and growth by impacting the AKT1 and VEGFA proteins. Molecular docking, complemented by a 20-ns molecular dynamic (MD) simulation, was used, and the binding affinity of the compound was further validated. Molecular docking, complemented by a 20-ns MD simulation, confirmed the binding affinity of these compounds. Specifically, for AKT1, ascorbic acid showed a docking score of -11.1227 kcal/mol, interacting with residues Ser A:240, Leu A:239, Arg A:243, Arg C:2, and Glu A:341. For VEGFA, hispidulin exhibited a docking score of -17.3714 kcal/mol, interacting with Asn A:158, Val A:190, Gln B:160, Ser A:179, and Ser B:176. To sum up, both a theoretical and empirical framework were established by this study, directing more comprehensive research and laying out a roadmap for the potential utilization of active compounds in the formulation of anti-cancer treatments.
Calcium l-Malate and d-Tartarate Frameworks as Adjuvants for the Sustainable Delivery of a Fungicide.[Pubmed:38109287]
ACS Appl Mater Interfaces. 2023 Dec 18.
Agrichemical adjuvants that combine a highly selective, efficient, and active mode of operation are critically needed to realize a more sustainable approach to their usage. Herein, we report the synthesis and full characterization of two new metal-organic frameworks (MOFs), termed UPMOF-1 and UPMOF-2, that were constructed from eco-friendly Ca(2+) ions and naturally occurring, low-molecular weight plant acids, l-malic and D-Tartaric acid, respectively. Upon structural elucidation of both MOFs, a widely used fungicide, hexaconazole (Hex), was loaded on the structures, reaching binding affinities of -5.0 and -3.5 kcal mol(-1) and loading capacities of 63% and 62% for Hex@UPMOF-1 and Hex@UPMOF-2, respectively, as a result of the formation of stable host-guest interactions. Given the framework chemistry of the MOFs and their predisposition to disassembly under relevant agricultural conditions, the sustained release kinetics were determined to show nearly quantitative release (98% and 95% for Hex@UPMOF-1 and Hex@UPMOF-2, respectively) after >500 h, a release profile drastically different than the control (>80% release in 24 h), from which the high efficiency of these new systems was established. To confirm their high selectivity and activity, in vitro and in vivo studies were performed to illustrate the abilities of Hex@UPMOF-1 and Hex@UPMOF-2 to combat the known aggressive pathogen Ganoderma boninense that causes basal stem rot disease in oil palm. Accordingly, at an extremely low concentration of 0.05 mug mL(-1), both Hex@UPMOF-1 and Hex@UPMOF-2 were demonstrated to completely inhibit (100%) G. boninense growth, and during a 26 week in vivo nursery trial, the progression of basal stem rot infection was completely halted upon treatment with Hex@UPMOF-1 and Hex@UPMOF-2 and seedling growth was accelerated given the additional nutrients supplied via the disassembly of the MOFs. This study represents a significant step forward in the design of adjuvants to support the environmentally responsible use of agrichemical crop protection.
Byproducts of inflammatory radical metabolism provide transient nutrient niches for microbes in the inflamed gut.[Pubmed:38106073]
bioRxiv [Preprint]. 2023 Dec 8:2023.12.08.570695.
Louis Pasteur's experiments on tartaric acid laid the foundation for our understanding of molecular chirality, but major questions remain. By comparing the optical activity of naturally-occurring tartaric acid with chemically-synthesized paratartaric acid, Pasteur realized that naturally-occurring tartaric acid contained only L-tartaric acid while paratartaric acid consisted of a racemic mixture of D- and L-tartaric acid. Curiously, D-Tartaric acid has no known natural source, yet several gut bacteria specifically degrade D-Tartaric acid. Here, we investigated the oxidation of monosaccharides by inflammatory reactive oxygen and nitrogen species. We found that this reaction yields an array of alpha hydroxy carboxylic acids, including tartaric acid isomers. Utilization of inflammation- derived D- and L-tartaric acid enhanced colonization by Salmonella Typhimurium and E. coli in murine models of gut inflammation. Our findings suggest that byproducts of inflammatory radical metabolism, such as tartrate and other alpha hydroxy carboxylic acids, create transient nutrient niches for enteric pathogens and other potentially harmful bacteria. Furthermore, this work illustrates that inflammatory radicals generate a zoo of molecules, some of which may erroneously presumed to be xenobiotics.
Enantioselective Oral Absorption of Molecular Chiral Mesoporous Silica Nanoparticles.[Pubmed:37839052]
Adv Mater. 2023 Dec;35(49):e2307900.
Inspired by the unique pharmacological effects of chiral drugs in the asymmetrical body environments, it is assumed that the chirality of nanocarriers is also a key factor to determine their oral adsorption efficiency, apart from their size, shape, etc. Herein, l/D-Tartaric acid modified mesoporous silica nanoparticles (l/d-CMSNs) are fabricated via a one-pot cocondensation method, and focused on whether the oral adsorption of nanocarriers will be benefited from their chirality. It is found that l-CMSN performed better in the sequential oral absorption processes, including mucus permeation, mucosa bio-adhesion, cellular uptake, intestinal transport and gastrointestinal tract (GIT) retention, than those of the d-chiral (d-CMSN), racemic (dl-CMSN), and achiral (MSN) counterparts. The multiple chiral recognition mechanisms are experimentally and theoretically demonstrated following simple differential adsorption on biointerfaces, wherein electrostatic interaction is the dominant energy. During the oral delivery task, l-CMSN, which is proven to be stable, nonirritative, biocompatible, and biodegradable, is efficiently absorbed into the blood (1.72-2.05-fold higher than other nanocarriers), and helps the loaded doxorubicin (DOX) to achieve better intestinal transport (2.32-27.03-times higher than other samples), satisfactory bioavailability (449.73%) and stronger antitumor effect (up to 95.43%). These findings validated the dominant role of chirality in determining the biological fate of nanocarriers.
Optical Asymmetry and Structural Complexity in Hierarchically Organized Chiral CuO Nanostructures: Insight into the Geometric and Crystallographic Effects on Cooperative Chirality.[Pubmed:37768369]
Inorg Chem. 2023 Oct 16;62(41):16725-16733.
Optical asymmetry and structural complexity across different length scales were realized in flower-shaped CuO nanostructures, prepared through refluxing an aqueous solution of copper acetate, sodium hydroxide, and D-Tartaric acid, as well as in their toroid-like forms obtained on calcination at 600 degrees C. Atomic scale chirality in the flower morphology could be visualized as putative Boerdijk-Coexter-Bernal like tetrahelical fragments, while that in the toroid form could be identified as screw dislocation-driven helicity. The fraction of asymmetry in the nanostructures has been evaluated from their chiroptical responses based on Kuhn asymmetry factor (g) from circular dichroism (CD) spectroscopy in the entire UV-vis range. The origin of chirality in the two CuO nanostructures has been assigned to the helical arrangement of the Cu-O-Cu network in accordance with their microscopic and spectroscopic observations. Attempts have been made to interpret the crystallographic and geometric chiralities in the two CuO nanostructures based on the redshift and augmented intensity of the CD signal along with an increase in their corresponding anisotropic factor on calcination. Further, the diverse interaction of the toroid-shaped CuO nanostructures with enantiomeric tryptophan moieties has been illustrated from the measurement of their corresponding thermodynamic parameters.
D-tartaric acid doping improves the performance of whole-cell bacteria imprinted polymer for sensing Vibrio parahaemolyticus.[Pubmed:37524461]
Anal Chim Acta. 2023 Sep 22;1275:341567.
Whole-cell bacteria imprinted polymer-based sensors still face challenges in the form of the difficulty of removing the template entirely, low affinity, and poor sensitivity. To further improve their performance, it is pivotal to modulate the morphology and chemical properties of imprintied polymer by taking advantage of doping engineering. Here we introduced D-Tartaric acid (D-TA) as a dopant and employed pyrrole as a functional monomer to construct D-TA/polypyrrole (PPy)-based bacteria imprinted polymer (DPBIP) sensor for Vibrio parahaemolyticus (VP) detection. It is demonstrated that D-TA doping can synergistically accelerate the removal of template bacteria from imprinted polymers (1.5 h), improve bacteria affinity of imprinted sites (the recognition time of 30 min), and enhance the sensitivity of DPBIP sensor (a detection limit of 19 CFU mL(-1)). The DPBIP sensor had a linear range of 10(2) approximately 10(6) CFU mL(-1) and exhibited high selectivity and good repeatability. Moreover, a recovery of 94.8%-105.3% was achieved in drinking water and oyster samples. Therefore, small functional molecules doping opens a new avenue to engineering BIP-based sensors with high performance, holding potential applications in securing food safety.
Chiral-Controlled Cyclic Chemiluminescence Reactions for the Analysis of Enantiomer Amino Acids.[Pubmed:37068187]
Anal Chem. 2023 May 2;95(17):6971-6979.
The similarity and complexity of chiral amino acids (AAs) in complex samples remain a significant challenge in their analysis. In this work, the chiral metal-organic framework (MOF)-controlled cyclic chemiluminescence (CCL) reaction is developed and utilized in the analysis of enantiomer AAs. The chiral MOF of d-Co(0.75)Zn(0.25)-MOF-74 is designed and prepared by modifying the Co(0.75)Zn(0.25)-MOF-74 with D-Tartaric acid. The developed chiral bimetallic MOF can not only offer the chiral recognize sites but also act as the catalyst in the cyclic luminol-H(2)O(2) reaction. Moreover, a distinguishable CCL signal can be obtained on enantiomer AAs via the luminol-H(2)O(2) reaction with the control of d-Co(0.75)Zn(0.25)-MOF-74. The amplified difference of enantiomer AAs can be quantified by the decay coefficient (k-values) which are calculated from the exponential decay fitting of their obtained CCL signals. According to simulation results, the selective recognition of 19 pairs of AAs is controlled by the pore size of the MOF-74 and their hydrogen-bond interaction with D-Tartaric acid on the chiral MOF. Furthermore, the k-values can also be used to estimate the change of chiral AAs in complex samples. Consequently, this chiral MOF-controlled CCL reaction is applied to differentiate enantiomer AAs involved in the quality monitoring of dairy products and auxiliary diagnosis, which provides a new approach for chiral studies and their potential applications.
Novel multi-component crystals of berberine with improved pharmaceutical properties.[Pubmed:36598503]
IUCrJ. 2023 Jan 1;10(Pt 1):66-76.
As an extremely popular natural product, berberine (BER) is mainly used for gastroenteritis and diarrhoea caused by bacteria. Research has also revealed the potent and extensive pharmacological properties of BER including its anti-arrhythmic, anti-tumour, anti-inflammatory and hypoglycemic activities and so on; therefore, BER is a promising drug for further development. However, its commercial form with hydrochloride exhibits poor stability and solubility, which are detrimental to its clinical therapeutic effects. For these purposes, the salt form was regulated via the reactive crystallization of 8-hydroxy-7,8-dihydroberberine (8H-HBER) with five pharmaceutically suitable organic acids including malonic acid (MA), L-tartaric acid (LTA), D-Tartaric acid (DTA), DL-tartaric acid (DLTA) and citric acid (CA), resulting in the six novel solid forms 1BER-1LTA-1W, 1BER-1DTA-1W, 1BER-1DLTA and 2BER-2CA as well as two rare multi-stoichiometric solid forms 1BER-1MA and 1BER-2MA-2W. The preparation of the multi-stoichiometric products was greatly influenced by both the crystallization solvent type and the molar ratio of reactants. The structures of these multi-component solid forms were determined using single-crystal X-ray diffraction and further characterized by powder X-ray diffraction, thermal analysis and Fourier transform infrared spectroscopy. Stability experiments showed that all samples prepared had superior physical stability under high temperature and high humidity. Furthermore, dissolution experiments demonstrated that the maximum apparent solubilities (MAS) of all the products were significantly improved compared with the commercial form of BER in dilute hydrochloric solution (pH = 1.2). In particular, the MAS of 1BER-1MA in dilute hydrochloric solution is as high as 34 times that of the commercial form. In addition, it is preliminarily confirmed that the MAS of the samples prepared in pure water and dilute hydrochloric solution is primarily influenced by a combination of factors including the packing index, intermolecular interactions, affinity of the counter-ion to the solvent, the molar ratio of the drug to counter-ion in the product and the common ion effect. These novel solids are potential candidates for BER solid forms with improved oral dosage design and may prompt further development.
Computer-Based Identification of Potential Druggable Targets in Multidrug-Resistant Acinetobacter baumannii: A Combined In Silico, In Vitro and In Vivo Study.[Pubmed:36296249]
Microorganisms. 2022 Oct 5;10(10):1973.
The remarkable rise in antimicrobial resistance is alarming for Acinetobacter baumannii, which necessitates effective strategies for the discovery of promising anti-acinetobacter agents. We used a subtractive proteomics approach to identify unique protein drug targets. Shortlisted targets passed through subtractive channels, including essentiality, non-homology to the human proteome, druggability, sub-cellular localization prediction and conservation. Sixty-eight drug targets were shortlisted; among these, glutamine synthetase, dihydrodipicolinate reductase, UDP-N-acetylglucosamine acyltransferase, aspartate 1-decarboxylase and bifunctional UDP-N-acetylglucosamine diphosphorylase/glucosamine-1-phosphate N-acetyltransferase were evaluated in vitro by determining the minimum inhibitory concentration (MIC) of candidate ligands, citric acid, dipicolinic acid, D-Tartaric acid, malonic acid and 2-(N-morpholino)ethanesulfonic acid (MES), respectively, which ranged from 325 to 1500 mug/mL except for MES (25 mg/mL). The candidate ligands, citric acid, D-Tartaric acid and malonic acid, showed good binding energy scores to their targets upon applying molecular docking, in addition to a significant reduction in A. baumannii microbial load in the wound infection mouse model. These ligands also exhibited good tolerability to human skin fibroblast. The significant increase in the MIC of malonic acid in beta-alanine and pantothenate-supplemented media confirmed its selective inhibition to aspartate 1-decarboxylase. In conclusion, three out of sixty-eight potential A. baumannii drug targets were effectively inhibited in vitro and in vivo by promising ligands.
Impact of Weissella cibaria BYL4.2 and its supernatants on Penicillium chrysogenum metabolism.[Pubmed:36274712]
Front Microbiol. 2022 Oct 5;13:983613.
Lactic acid bacteria (LAB) can produce a vast spectrum of antifungal metabolites to inhibit fungal growth. The purpose of this study was to elucidate the antifungal effect of isolated Weissella cibaria BYL4.2 on Penicillium chrysogenum, the antifungal activity of W. cibaria BYL4.2 against P. chrysogenum was evaluated by the superposition method, results showed that it had obviously antifungal activity against P. chrysogenum. Studying the probiotic properties of BYL4.2 and determining it as beneficial bacteria. Furtherly, different treatments were carried out to characterize the antifungal activity of cell-free supernatant (CFS) produced by W. cibaria BYL4.2, and it was shown that the CFS was pH-dependent, partly heat-sensitive, and was not influenced by proteinaceous treatment. The CFS of W. cibaria BYL4.2 was analyzed by high-performance liquid chromatography (HPLC) and found the highest content of lactic acid. Screening of metabolic markers by a non-targeted metabolomics approach based liquid chromatography-mass spectrometry (LC-MS). The results speculated that organic acid especially detected D-Tartaric acid was the main antifungal substance of CFS, which could cause the down-regulation of metabolites in the ABC transporters pathway, thereby inhibiting the growth of P. chrysogenum. Therefore, this study may provide important information for the inhibitory mechanism of W. cibaria BYL4.2 on P. chrysogenum, and provide a basis for further research on the antifungal effect of Weissella.
Formation and Transformation of Polystyrene-block-poly(2-vinylpyridine) Hexasomes in the Solvent Exchange.[Pubmed:36196878]
Langmuir. 2022 Oct 18;38(41):12441-12449.
The generation of inverse micellar nanostructures, especially those with open channels, using commercially available diblock copolymers (BCP), is vital for their wide applications in drug delivery and catalyst templating. However, the rigid requirements for forming inverse morphologies, such as the highly asymmetric molecular structures, the semicrystalline motifs, and concentrated solutions of diblock copolymers, represent obstacles to the development of successful strategies. In this study, the inverse polystyrene-block-poly(2-vinylpyridine) (PS(30K)-b-P2VP(8.5K)) micelles, i.e., the hexasomes with p6mm lattice, were generated through a modified solvent exchange via adding D-Tartaric acid (d-TA) in the nonsolvent. Various intermediate morphologies have been identified with the change of d-TA concentration. Interestingly, in the high d-TA concentration ( approximately 20 mg/mL), the hexasomes with close-packed hoops changed to mesoporous spheres with regularly packed perpendicular cylindrical channels (V(D-TA): V(BCP) 6:100), and further to the mesoporous spheres with gyri-like open pores (V(D-TA): V(BCP) > 15:100) with the increasing acidity in the mixed solvent. This study presents a simple and economical pathway for fabricating PS(30K)-b-P2VP(8.5K) hexasomes and first demonstrates these hexasomes can be modified to the morphology with open channels that will benefit their further applications.
Six-Pointed Star Chiral Cobalt Superstructures with Strong Antibacterial Activity.[Pubmed:36038354]
Small. 2022 Sep;18(39):e2204219.
Chiral inorganic nanomaterials have shown promise as a potential means of combating bacteria due to their high levels of biocompatibility, easy surface modification, and excellent optical properties. In this study, a diverse range of chiral hierarchical nanomaterials are prepared from Co(2+) and L/D-Tartaric acid (Tar) ligands. By combining the ligands in different ratios, chiral Co superstructures (Co SS) are obtained with different morphologies, including chiral nanoflowers, chiral nanohanamaki, a chiral six-pointed star, a chiral fan shape, and a chiral fusiform shape. It is found that the chiral six-pointed star structures exhibit chiroptical activity across a broad range of wavelengths from 300 to 1300 nm and that the g-factor is as high as 0.033 with superparamagnetic properties. Under the action of electromagnetic fields, the chiral six-pointed star Co SS shows excellent killing ability against Gram-positive Staphylococcus aureus (ATCC 25923). Compared to L-Co SS, D-Co SS shows stronger levels of antibacterial ability. It is found that the levels of reactive oxygen species generated by D-Co SS are 1.59-fold higher than L-Co SS which is attributed to chiral-induced spin selectivity effects. These findings are of significance for the further development of chiral materials with antibacterial properties.


