D-CPP-eneCAS# 117414-74-1 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Monomethyl auristatin E
Catalog No.:BCC1775
CAS No.:474645-27-7
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 117414-74-1 | SDF | Download SDF |
| PubChem ID | 6435801 | Appearance | Powder |
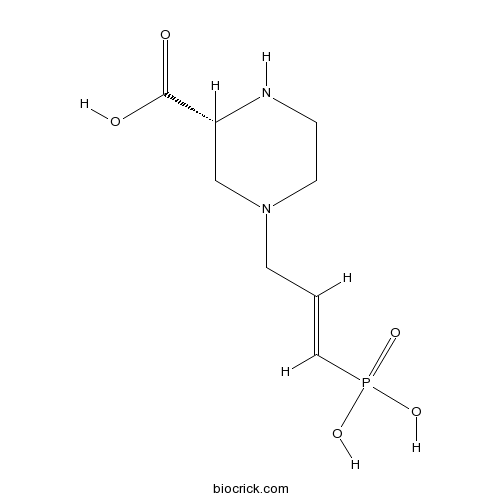
| Formula | C8H15N2O5P | M.Wt | 250.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Midafotel, SDZ EAA 494 | ||
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | (2R)-4-[(E)-3-phosphonoprop-2-enyl]piperazine-2-carboxylic acid | ||
| SMILES | C1CN(CC(N1)C(=O)O)CC=CP(=O)(O)O | ||
| Standard InChIKey | VZXMZMJSGLFKQI-ABVWVHJUSA-N | ||
| Standard InChI | InChI=1S/C8H15N2O5P/c11-8(12)7-6-10(4-2-9-7)3-1-5-16(13,14)15/h1,5,7,9H,2-4,6H2,(H,11,12)(H2,13,14,15)/b5-1+/t7-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and competitive NMDA antagonist (Ki = 40 nM). Centrally active following systemic administration. |

D-CPP-ene Dilution Calculator

D-CPP-ene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.997 mL | 19.9848 mL | 39.9696 mL | 79.9392 mL | 99.9241 mL |
| 5 mM | 0.7994 mL | 3.997 mL | 7.9939 mL | 15.9878 mL | 19.9848 mL |
| 10 mM | 0.3997 mL | 1.9985 mL | 3.997 mL | 7.9939 mL | 9.9924 mL |
| 50 mM | 0.0799 mL | 0.3997 mL | 0.7994 mL | 1.5988 mL | 1.9985 mL |
| 100 mM | 0.04 mL | 0.1998 mL | 0.3997 mL | 0.7994 mL | 0.9992 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-O-beta-Glucopyranosyl-cis-coumaric acid
Catalog No.:BCN1608
CAS No.:117405-48-8
- AZD2461
Catalog No.:BCC2214
CAS No.:1174043-16-3
- Dimethylwulignan A1
Catalog No.:BCN3624
CAS No.:117404-43-0
- Artoheterophyllin B
Catalog No.:BCN6050
CAS No.:1174017-37-8
- Endothelin 3 (human, rat)
Catalog No.:BCC5713
CAS No.:117399-93-6
- AZD6482
Catalog No.:BCC2523
CAS No.:1173900-33-8
- 2-Hydroxysaclofen
Catalog No.:BCC6579
CAS No.:117354-64-0
- 9,9-Bis[4-(2-hydroxyethoxy)phenyl]fluorene
Catalog No.:BCC8796
CAS No.:117344-32-8
- Emeheterone
Catalog No.:BCN7285
CAS No.:117333-12-7
- Clocinnamox mesylate
Catalog No.:BCC5684
CAS No.:117332-69-1
- PKI-402
Catalog No.:BCC3843
CAS No.:1173204-81-3
- U0126-EtOH
Catalog No.:BCC1066
CAS No.:1173097-76-1
- 2-Methyl-6-(p-tolyl)heptane-2,3-diol
Catalog No.:BCN7249
CAS No.:117421-22-4
- Xenin 8
Catalog No.:BCC5876
CAS No.:117442-28-1
- Wilforol E
Catalog No.:BCN8058
CAS No.:117456-86-7
- Triptonodiol
Catalog No.:BCN6782
CAS No.:117456-87-8
- BCECF-AM
Catalog No.:BCC5969
CAS No.:117464-70-7
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Prionitin
Catalog No.:BCN4855
CAS No.:117469-56-4
- Sesamoside
Catalog No.:BCN6051
CAS No.:117479-87-5
- ROX NHS ester, pure 6- isomer
Catalog No.:BCC3587
CAS No.:117491-83-5
- Neuromedin U (rat)
Catalog No.:BCC5847
CAS No.:117505-80-3
- Ustusol A
Catalog No.:BCN7719
CAS No.:1175543-02-8
- 2alpha,9alpha,11-Trihydroxy-6-oxodrim-7-ene
Catalog No.:BCN7741
CAS No.:1175543-03-9
The excitatory amino acid antagonist D-CPP-ene (SDZ EAA-494) in patients with epilepsy.[Pubmed:8269915]
Epilepsy Res. 1993 Oct;16(2):165-74.
The amino acids L-glutamate and L-aspartate have been shown to be excitatory neurotransmitters in mammalian central nervous systems. Antagonists acting selectively at excitatory amino acid receptors have shown antiepileptic properties in several animal models. We report the results of the first therapeutic trial of the competitive NMDA antagonist, D-CPP-ene (SDZ EAA-494), in eight patients with intractable complex partial seizures. All patients withdrew prematurely because of side-effects, including poor concentration (8), sedation (7), ataxia (6), depression (3), dysarthria (2), amnesia (2) and unilateral choreo-athetosis in a patient with contralateral Sturge-Weber syndrome. Seizures were unchanged in four patients and worse in three. A further patient with apparent improvement in seizures in the first week developed complex partial status epilepticus on withdrawal of DCPP-ene. EEG on treatment (5) or in the immediate post-treatment period (2) showed slowing of background activity and, in five cases, an increase in epileptiform activity. Serum concentrations of DCPP-ene were found to be unpredictable and higher than expected from pharmacokinetic data on normal subjects. There was no clear relationship between serum concentrations and the severity of side-effects. Preliminary experience with DCPP-ene in patients with refractory partial seizures is not promising. Evaluation of related compounds is warranted.
Effects of continual intravenous posttreatment with D-CPP-ene, a potent competitive N-methyl-D-aspartate receptor antagonist, on rat brain edema induced by injection of triethyltin into the cerebral hemisphere.[Pubmed:7675315]
Neurosci Lett. 1995 Jun 9;192(2):109-12.
Brain edema was produced by injecting triethyltin (TET) into the right cerebral hemisphere via the internal carotid artery in rats. TET induced a dose-related increase in mortality rate and brain water content. Immediately after TET-injection (2 mg/head), saline, glycerol (125 mg/ml) or the N-methyl-D-aspartate receptor antagonist (R)-4-(3-Phosphono-2-propenyl)-2-piperazine carboxylic acid (D-CPP-ene; 0.083 and 0.25 mg/ml) was continually infused via the right internal jugular vein at 20 microliters/min for 6 h. The mortality rate and brain water content were significantly decreased after infusion of 0.25 mg/ml D-CPP-ene, but only somewhat reduced after glycerol infusion when compared with the saline group. The results suggest that continual intravenous posttreatment with D-CPP-ene is useful for treatment of brain edema.
Focal cerebral ischemia in the cat: pretreatment with a competitive NMDA receptor antagonist, D-CPP-ene.[Pubmed:2166743]
J Cereb Blood Flow Metab. 1990 Sep;10(5):668-74.
The effects of the competitive N-methyl-D-aspartate (NMDA) receptor antagonist D-(E)-4-(3-phosphonoprop-2-enyl)piperazine-2-carboxylic acid (D-CPP-ene; SDZ EAA 494) upon ischemic brain damage have been examined in anesthetized cats. Focal cerebral ischemia was produced by permanent occlusion of the middle cerebral artery (MCA) and the animals were killed 6 h later. The amount of early ischemic brain damage was assessed in coronal sections at 16 predetermined stereotaxic planes. Pretreatment with D-CPP-ene (15 mg/kg i.v. followed by continuous infusion at 0.17 mg/kg/min until death), 15 min prior to MCA occlusion, significantly reduced the volume of ischemic brain damage (from 20.6 +/- 9.9% of the cerebral hemisphere in vehicle-treated cats to 7.2 +/- 4.4% in drug-treated cats; p less than 0.01). The competitive NMDA receptor antagonist D-CPP-ene is as effective as noncompetitive NMDA antagonists in reducing the amount of ischemic brain damage in this model of focal cerebral ischemia in a gyrencephalic species.
The competitive NMDA antagonist, D-CPP-ene, potentiates the anticonvulsant activity of conventional antiepileptics against maximal electroshock-induced seizures in mice.[Pubmed:7936096]
Neuropharmacology. 1994 May;33(5):619-24.
D-CPP-ene[3-(2-carboxy-piperazine-4-yl)-1-propenyl-1-phosphonic acid; a competitive antagonist of N-methyl-D-aspartic acid] in a dose of 2 mg/kg (i.p.) significantly increased the threshold for electroconvulsions. When given in a dose half that affecting the electroconvulsive threshold, D-CPP-ene potentiated the anticonvulsant activity of carbamazepine, diazepam, diphenylhydantoin, phenobarbital and valproate against maximal electroshock (50 mA)-induced seizures in mice. However, this NMDA antagonist did not influence the plasma levels of the antiepileptic drugs studied, so a pharmacokinetic interaction, in terms of total plasma levels at least, is not probable. The chimney test and retention test in mice revealed that the combined treatment of D-CPP-ene at 1.0 mg/kg (i.p.) with either diazepam, diphenylhydantoin, phenobarbital or valproate (providing a 50% protection against maximal electroshock convulsions) resulted in motor impairment and caused impairment of long-term memory. On the other hand, a combination of D-CPP-ene and carbamazepine was devoid of adverse effects. It can be concluded that the potential utility of D-CPP-ene in combination with conventional antiepileptic drugs does not seem promising, except for carbamazepine.
Effects of the NMDA receptor antagonist D-CPPene on extracellular levels of dopamine and dopamine and serotonin metabolites in striatum of kindled and non-kindled rats.[Pubmed:10422758]
Eur J Pharmacol. 1999 Jun 18;374(2):175-87.
Electrical kindling in rats has previously been shown to cause a hypersensitivity to amphetamine-like behavioral effects of competitive NMDA receptor antagonists such as D,L-(E)-amino-4-methyl-5-phosphono-3-pentenoic acid (CGP 37849), D-(E)-2-amino-4-methyl-5-phosphono-3-pentenoic acid (CGP 40116), or 3-(2-carboxypiperazine-4-yl)propenyl-1-phosphonate (SDZ EAA 494; D-CPPene). From this observation, it was concluded that kindling-induced epileptogenesis enhances the potential of competitive NMDA receptor antagonists to induce such unwanted adverse effects, predicting that such drugs may induce more severe side effects in epileptic patients than in healthy volunteers, which was confirmed in clinical trials. In the present study, we thought to examine the biochemical basis for the enhanced susceptibility of kindled rats to amphetamine-like behavioral effects of NMDA receptor antagonists by measuring extracellular levels of dopamine, the dopamine metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), and the serotonin (5-hydroxytryptamine, 5-HT) metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the striatum of awake, behaving rats, using in vivo microdialysis. When administered systemically, D-CPPene, 15 mg/kg i.p., caused more intense stereotyped behaviors in kindled than in non-kindled rats. While there was no significant alteration in extracellular dopamine, in both groups of rats HVA and 5-HIAA significantly increased. In kindled rats, basal levels of HVA and the increase in HVA in response to D-CPPene were higher compared to non-kindled animals. When administered intrastriatally via the microdialysis probe, D-CPPene, 10 microM, significantly increased dopamine, HVA and 5-HIAA, which was associated with stereotyped behaviors. Again, these behaviors were more intense in kindled rats. The data indicate that a competitive NMDA receptor antagonist at high, behaviorally active doses induces increases in striatal dopamine and presumably also 5-HT release, which most likely underlie the amphetamine-like behavioral effects of such a drug. Kindling enhances the sensitivity to these behavioral effects, which could be related to a more marked dopamine and 5-HT release. Thus, in order to avoid false predictions for the clinical situation, it is important to study the behavioral and biochemical effects of NMDA receptor antagonists not only in naive, healthy animals but also in animals that mimic the disease for which a drug is developed.
D-CPP-ene (SDZ EAA 494), a potent and competitive N-methyl-D-aspartate (NMDA) antagonist: effect on spontaneous activity and NMDA-induced depolarizations in the rat neocortical slice preparation, compared with other CPP derivatives and MK-801.[Pubmed:2166255]
Neurosci Lett. 1990 Jun 8;113(3):315-21.
D- and L-enantiomers of the competitive NMDA antagonists CPP and CPP-ene, as well as the non-competitive NMDA antagonist MK-801, inhibited spontaneous activity occurring in slices of rat cerebral cortex exposed to Mg2(+)-free medium. D-CPP-ene (SDZ EAA 494) was the most active competitive antagonist with a threshold concentration of 10 nM and an ED50 of 39 nM. The inhibitory effects of all competitive antagonists were reversible, whereas reversibility following MK-801 (ED50 = 33 nM) was incomplete and slow. D-CPP-ene was also the most potent competitive antagonist against NMDA-evoked depolarizations, having an apparent pA2 value of 6.8; its action was specific to the NMDA type of excitatory amino acid receptor.


