CytosineCAS# 71-30-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 71-30-7 | SDF | Download SDF |
| PubChem ID | 597 | Appearance | Powder |
| Formula | C4H5N3O | M.Wt | 111.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 16.67 mg/mL (150.05 mM; Need ultrasonic) | ||
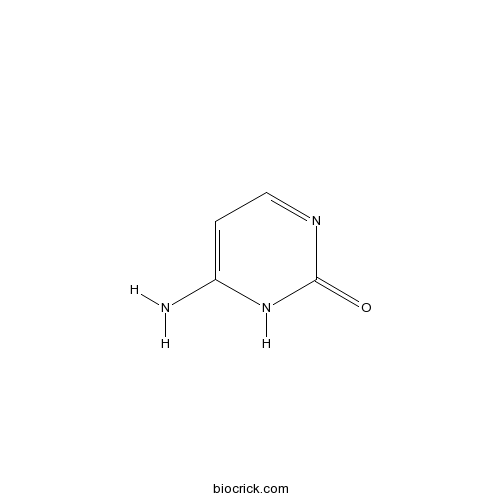
| Chemical Name | 6-amino-1H-pyrimidin-2-one | ||
| SMILES | C1=C(NC(=O)N=C1)N | ||
| Standard InChIKey | OPTASPLRGRRNAP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H5N3O/c5-3-1-2-6-4(8)7-3/h1-2H,(H3,5,6,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cytosine Dilution Calculator

Cytosine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 9.0009 mL | 45.0045 mL | 90.009 mL | 180.018 mL | 225.0225 mL |
| 5 mM | 1.8002 mL | 9.0009 mL | 18.0018 mL | 36.0036 mL | 45.0045 mL |
| 10 mM | 0.9001 mL | 4.5005 mL | 9.0009 mL | 18.0018 mL | 22.5023 mL |
| 50 mM | 0.18 mL | 0.9001 mL | 1.8002 mL | 3.6004 mL | 4.5005 mL |
| 100 mM | 0.09 mL | 0.45 mL | 0.9001 mL | 1.8002 mL | 2.2502 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-His-OH
Catalog No.:BCC2954
CAS No.:71-00-1
- 7,3',4'-Tri-O-methyleriodictyol
Catalog No.:BCN7766
CAS No.:70987-96-1
- 2-(5-Chloro-2-phenoxyphenyl)acetic acid
Catalog No.:BCC8481
CAS No.:70958-20-2
- SirReal2
Catalog No.:BCC6513
CAS No.:709002-46-0
- Dihydrocitflavanone
Catalog No.:BCN6873
CAS No.:70897-14-2
- (2S)-Isoxanthohumol
Catalog No.:BCN2892
CAS No.:70872-29-6
- Troxerutin
Catalog No.:BCN3828
CAS No.:7085-55-4
- Chlorothiazide Sodium
Catalog No.:BCC5628
CAS No.:7085-44-1
- Cyanidin-3-O-glucoside chloride
Catalog No.:BCN1230
CAS No.:7084-24-4
- Chicoric acid
Catalog No.:BCN1215
CAS No.:70831-56-0
- JNJ 10397049
Catalog No.:BCC6139
CAS No.:708275-58-5
- Panaxydiol
Catalog No.:BCN3702
CAS No.:708257-91-4
- butanol
Catalog No.:BCN4976
CAS No.:71-36-3
- Medroxyprogesterone acetate
Catalog No.:BCC4485
CAS No.:71-58-9
- Veratridine
Catalog No.:BCC7515
CAS No.:71-62-5
- Digitoxin
Catalog No.:BCN5358
CAS No.:71-63-6
- Cathinone
Catalog No.:BCN1784
CAS No.:71031-15-7
- 1-Octacosanoyl glyceride
Catalog No.:BCN8190
CAS No.:71035-02-4
- Griselinoside
Catalog No.:BCN4272
CAS No.:71035-06-8
- 5,8,4'-Trihydroxy-7-methoxyflavone 8-O-glucoside
Catalog No.:BCN1372
CAS No.:710952-13-9
- MRS 2578
Catalog No.:BCC4976
CAS No.:711019-86-2
- Bucindolol
Catalog No.:BCC7444
CAS No.:71119-11-4
- Meloxicam (Mobic)
Catalog No.:BCC3808
CAS No.:71125-38-7
- (E)-3-Acetoxy-5-methoxystilbene
Catalog No.:BCN4273
CAS No.:71144-78-0
Molybdenum.[Pubmed:29767695]
Adv Nutr. 2018 May 1;9(3):272-273.
Molybdenum, a trace element essential for micro-organisms, plants, and animals, was discovered in 1778 by a Swedish chemist named Karl Scheele. Initially mistaken for lead, molybdenum was named after the Greek work molybdos, meaning lead-like. In the 1930s, it was recognized that ingestion of forage with high amounts of molybdenum by cattle caused a debilitating condition. In the 1950s, the essentiality of molybdenum was established with the discovery of the first molybdenum-containing enzymes. In humans, only 4 enzymes requiring molybdenum have been identified to date: sulfite oxidase, xanthine oxidase, aldehyde oxidase, and mitochondrial amidoxime-reducing component (mARC). Sulfite oxidase, an enzyme found in mitochondria, catalyzes oxidation of sulfite to sulfate, the final step in oxidation of sulfur amino acids (cysteine and methionine). Xanthine oxidase converts hypoxanthine to xanthine, and further converts xanthine to uric acid, preventing hypoxanthine, formed from spontaneous deamination of adenine, from leading to DNA mutations if paired with Cytosine in place of thymine. Aldehyde oxidase is abundant in the liver and is an important enzyme in phase 1 drug metabolism. Finally, mARC, discovered less than a decade ago, works in concert with cytochrome b5 type B and NAD(H) cytochrome b5 reductase to reduce a variety of N-hydroxylated substrates, although the physiologic significance is still unclear. In the case of each of the molybdenum enzymes, activity is catalyzed via a tricyclic cofactor composed of a pterin, a dithiolene, and a pyran ring, called molybdenum cofactor (MoCo) (1).
Lineage-associated underrepresented permutations (LAUPs) of mammalian genomic sequences based on a Jellyfish-based LAUPs analysis application (JBLA).[Pubmed:29762634]
Bioinformatics. 2018 Nov 1;34(21):3624-3630.
Motivation: This study addresses several important questions related to naturally underrepresented sequences: (i) are there permutations of real genomic DNA sequences in a defined length (k-mer) and a given lineage that do not actually exist or underrepresented? (ii) If there are such sequences, what are their characteristics in terms of k-mer length and base composition? (iii) Are they related to CpG or TpA underrepresentation known for human sequences? We propose that the answers to these questions are of great significance for the study of sequence-associated regulatory mechanisms, such Cytosine methylation and chromosomal structures in physiological or pathological conditions such as cancer. Results: We empirically defined sequences that were not included in any well-known public databases as lineage-associated underrepresented permutations (LAUPs). Then, we developed a Jellyfish-based LAUPs analysis application (JBLA) to investigate LAUPs for 24 representative species. The present discoveries include: (i) lengths for the shortest LAUPs, ranging from 10 to 14, which collectively constitute a low proportion of the genome. (ii) Common LAUPs showing higher CG content over the analysed mammalian genome and possessing distinct CG*CG motifs. (iii) Neither CpG-containing LAUPs nor CpG island sequences are randomly structured and distributed over the genomes; some LAUPs and most CpG-containing sequences exhibit an opposite trend within the same k and n variants. In addition, we demonstrate that the JBLA algorithm is more efficient than the original Jellyfish for computing LAUPs. Availability and implementation: We developed a Jellyfish-based LAUP analysis (JBLA) application by integrating Jellyfish (Marcais and Kingsford, 2011), MEME (Bailey, et al., 2009) and the NCBI genome database (Pruitt, et al., 2007) applications, which are listed as Supplementary Material. Supplementary information: Supplementary data are available at Bioinformatics online.
Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC.[Pubmed:29762717]
Neuro Oncol. 2018 Sep 3;20(10):1383-1392.
Background: Vocimagene amiretrorepvec (Toca 511) is an investigational gamma-retroviral replicating vector encoding Cytosine deaminase that, when used in combination with extended-release 5-fluoroCytosine (Toca FC), results preclinically in local production of 5-fluorouracil, depletion of immune-suppressive myeloid cells, and subsequent induction of antitumor immunity. Recurrent high-grade glioma (rHGG) patients have a high unmet need for effective therapies that produce durable responses lasting more than 6 months. In this setting, relapse is nearly universal and most responses are transient. Methods: In this Toca 511 ascending-dose phase I trial (NCT01470794), HGG patients who recurred after standard of care underwent surgical resection and received Toca 511 injected into the resection cavity wall, followed by orally administered cycles of Toca FC. Results: Among 56 patients, durable complete responses were observed. A subgroup was identified based on Toca 511 dose and entry requirements for the follow-up phase III study. In this subgroup, which included both isocitrate dehydrogenase 1 (IDH1) mutant and wild-type tumors, the durable response rate is 21.7%. Median duration of follow-up for responders is 35.7+ months. As of August 25, 2017, all responders remain in response and are alive 33.9+ to 52.2+ months after Toca 511 administration, suggesting a positive association of durable response with overall survival. Conclusions: Multiyear durable responses have been observed in rHGG patients treated with Toca 511 + Toca FC in a phase I trial, and the treatment will be further evaluated in a randomized phase III trial. Among IDH1 mutant patients treated at first recurrence, there may be an enrichment of complete responders.
Effects of hydrophilic monomers on sorptive properties of divinylbenzene-based reversed phase sorbents.[Pubmed:29759223]
Talanta. 2018 Aug 1;185:427-432.
Solid phase extraction (SPE) has been extensively used as a pretreatment method. In SPE methods, commercially available reversed phase type sorbents, which consist of macroporus styrene-divinylbenzene or copolymers including divinylbenzene (DVB) and hydrophilic monomers, have been applied to a variety of samples. The later sorbents are called hydrophilic lipophilic balanced (HLB) type sorbents. Hydrophilic monomers in hydrophilic lipophilic balanced type sorbents contribute to the increase in retention of polar compounds, because hydrophilic monomers improve the wettability and increase the interaction with polar compounds as analytes. In this study, three different methacrylate monomers (ethylene glycol dimethacrylate (EGDMA), glycerol dimethacrylate (GDMA) and trimethylolpropane trimethacrylate (TMPTMA)), which are expected to improve the retention of polar compounds, were chosen, and DVB-based copolymetric sorbents including the three monomers were newly synthesized. Among them, the sorbents including GDMA or TMPTMA gave higher recoveries to polar compounds such as uridine and adenine than that including EGDMA. The optimization studies of hydrophilic lipophilic balance, inert diluent and the purity of DVB improved the sorptive abilities of the sorbents. The developed sorbents have higher recoveries for variety of polar compounds (Cytosine, uracil, cytidine, uridine, 2'-deoxycytidine, 2'-deoxyguanosine, adenine, thymidine, adenosine and 2'-deoxyadenosine) than commercially available hydrophilic lipophilic balanced type sorbents, while the recoveries for theophylline were comparable between the proposed sorbents and the commercial sorbents.
[Cloning,expression and activity identification of human innate immune protein apolipoprotein B mRNA editing enzyme catalytic subunit 3A(APOBEC3A)].[Pubmed:29762985]
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017 Feb;33(2):179-84.
Objective: To construct the expression vector of apolipoprotein B mRNA editing enzyme catalytic subunit 3A( APOBEC3A),express APOBEC3 A in eukaryotic cells and identify its Cytosine deaminase activity. Methods: The APOBEC3 A gene was obtained by PCR and inserted into the eukaryotic expression vector pc DNA3. 0( +). The recombinant vector pc DNA3. 0-APOBEC3 A was then transfected into HEK293 T and Hep G2 cells after confirmed by DNA sequencing. The recombinant protein was purified by Ni-NTA His Bind affinity column. Western blot analysis was used to detect the expression of APOBEC3 A protein. The localization of APOBEC3 A protein in HEK293 T and Hep G2 cel s was identified by immunofluorescence cytochemistry. The deaminase activity of APOBEC3 A protein was characterized by fluorescence polarization. Results: DNA sequencing confirmed that APOBEC3 A gene( 600 bp) was inserted into pc DNA3. 0-APOBEC3 A,which was expressed in HEK293 T and Hep G2 cells successfully. APOBEC3 A protein was mainly expressed in cytoplasm of HEK293 T cells and cytoplasm and nuclei of Hep G2 cells. APOBEC3 A protein showed Cytosine deaminase activity on the TTCA sequence in single-stranded DNA. Conclusion: The study constructed successfully APOBEC3 A eukaryotic expression vector,identified the differential expression of APOBEC3 A protein in HEK293 T and Hep G2 cells,and confirmed that the APOBEC3 A protein had Cytosine deaminase activity.
NMR analyses on N-hydroxymethylated nucleobases - implications for formaldehyde toxicity and nucleic acid demethylases.[Pubmed:29767200]
Org Biomol Chem. 2018 May 30;16(21):4021-4032.
Formaldehyde is produced in cells by enzyme-catalysed demethylation reactions, including those occurring on N-methylated nucleic acids. Formaldehyde reacts with nucleobases to form N-hydroxymethylated adducts that may contribute to its toxicity/carcinogenicity when added exogenously, but the chemistry of these reactions has been incompletely defined. We report NMR studies on the reactions of formaldehyde with canonical/modified nucleobases. The results reveal that hydroxymethyl hemiaminals on endocyclic nitrogens, as observed with thymidine and uridine monophosphates, are faster to form than equivalent hemiaminals on exocyclic nitrogens; however, the exocyclic adducts, as formed with adenine, guanine and Cytosine, are more stable in solution. Nucleic acid demethylase (FTO)-catalysed hydroxylation of (6-methyl)adenosine results in (6-hydroxymethyl)adenosine as the major observed product; by contrast no evidence for a stable 3-hydroxymethyl adduct was accrued with FTO-catalysed oxidation of (3-methyl)thymidine. Collectively, our results imply N-hydroxymethyled adducts of nucleic acid bases, formed either by reactions with formaldehyde or via demethylase catalysis, have substantially different stabilities, with some being sufficiently stable to have functional roles in disease or the regulation of nucleic acid/nucleobase activity.


