ColumbamineCAS# 3621-36-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3621-36-1 | SDF | Download SDF |
| PubChem ID | 72310 | Appearance | Powder |
| Formula | C20H20NO4 | M.Wt | 338.38 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Columbamin; Dehydroisocorypalmine | ||
| Solubility | DMSO : ≥ 23 mg/mL (67.97 mM) *"≥" means soluble, but saturation unknown. | ||
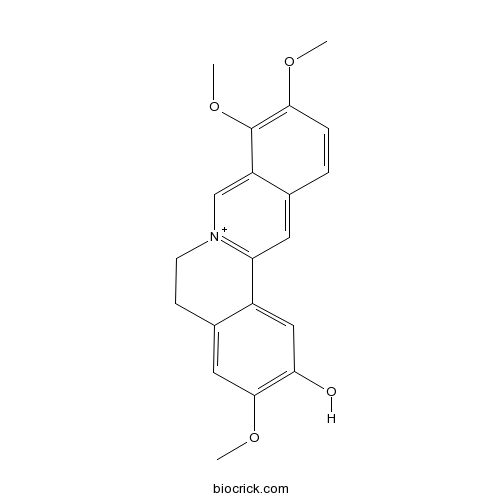
| Chemical Name | 3,9,10-trimethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium-2-ol | ||
| SMILES | COC1=C(C2=C[N+]3=C(C=C2C=C1)C4=CC(=C(C=C4CC3)OC)O)OC | ||
| Standard InChIKey | YYFOFDHQVIODOQ-UHFFFAOYSA-O | ||
| Standard InChI | InChI=1S/C20H19NO4/c1-23-18-5-4-12-8-16-14-10-17(22)19(24-2)9-13(14)6-7-21(16)11-15(12)20(18)25-3/h4-5,8-11H,6-7H2,1-3H3/p+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Columbamine exerts anti-proliferative and anti-vasculogenic effects on metastatic human osteosarcoma U2OS cells with low toxicity. It shows strong acetylcholinesterase (AChE) inhibitory activity with IC50 48.1 µM. |
| Targets | AChE | STAT | CDK | MMP(e.g.TIMP) |
| In vitro | Columbamine suppresses the proliferation and neovascularization of metastatic osteosarcoma U2OS cells with low cytotoxicity.[Pubmed: 23124089]Toxicol Lett. 2012 Dec 17;215(3):174-80.Columbamine (COL), an active component of the herb Coptis chinensis, inhibited the proliferation and neovascularization of metastatic osteosarcoma U2OS cells.
|
| Kinase Assay | Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa.[Pubmed: 24448061]Molecules. 2014 Jan 20;19(1):1201-11.Quaternary alkaloids are the major alkaloids isolated from Tinospora species. A previous study pointed to the necessary presence of quaternary nitrogens for strong acetylcholinesterase (AChE) inhibitory activity in such alkaloids.
|

Columbamine Dilution Calculator

Columbamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9553 mL | 14.7763 mL | 29.5526 mL | 59.1051 mL | 73.8814 mL |
| 5 mM | 0.5911 mL | 2.9553 mL | 5.9105 mL | 11.821 mL | 14.7763 mL |
| 10 mM | 0.2955 mL | 1.4776 mL | 2.9553 mL | 5.9105 mL | 7.3881 mL |
| 50 mM | 0.0591 mL | 0.2955 mL | 0.5911 mL | 1.1821 mL | 1.4776 mL |
| 100 mM | 0.0296 mL | 0.1478 mL | 0.2955 mL | 0.5911 mL | 0.7388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Columbamine is a quaternary isoquinoline alkaloid isolated from Argemone mexicana.
- NVP 231
Catalog No.:BCC4244
CAS No.:362003-83-6
- 2-Methoxyestradiol (2-MeOE2)
Catalog No.:BCC2228
CAS No.:362-07-2
- Genistein 7,4'-di-O-beta-D-glucopyranoside
Catalog No.:BCN7835
CAS No.:36190-98-4
- 3'-O-Methylorobol
Catalog No.:BCN5318
CAS No.:36190-95-1
- 3-Acetyl-2,5-dichlorothiophene
Catalog No.:BCC8602
CAS No.:36157-40-1
- Blumenol B
Catalog No.:BCN5317
CAS No.:36151-01-6
- Kobusin
Catalog No.:BCN7563
CAS No.:36150-23-9
- Dehydroleucodine
Catalog No.:BCN6897
CAS No.:36150-07-9
- Saxalin
Catalog No.:BCC8357
CAS No.:36150-06-8
- Mullilam diol
Catalog No.:BCN5316
CAS No.:36150-04-6
- Phytin
Catalog No.:BCN1285
CAS No.:3615-82-5
- alpha-L-Rhamnose
Catalog No.:BCN2592
CAS No.:3615-41-6
- Jatrorrhizine
Catalog No.:BCN5319
CAS No.:3621-38-3
- Cyclo(D-Leu-L-Pro)
Catalog No.:BCN4028
CAS No.:36238-67-2
- TC 1
Catalog No.:BCC7450
CAS No.:362512-81-0
- (±)-SLV 319
Catalog No.:BCC7954
CAS No.:362519-49-1
- Clemaphenol A
Catalog No.:BCN7834
CAS No.:362606-60-8
- Pitolisant
Catalog No.:BCC1862
CAS No.:362665-56-3
- Pitolisant oxalate
Catalog No.:BCC1864
CAS No.:362665-57-4
- Hederasaponin B
Catalog No.:BCN1085
CAS No.:36284-77-2
- Prostaglandin E2
Catalog No.:BCC7316
CAS No.:363-24-6
- Broussonol E
Catalog No.:BCN7996
CAS No.:363134-28-5
- Piroxicam
Catalog No.:BCC3841
CAS No.:36322-90-4
- YL-109
Catalog No.:BCC5543
CAS No.:36341-25-0
Columbamine suppresses the proliferation and neovascularization of metastatic osteosarcoma U2OS cells with low cytotoxicity.[Pubmed:23124089]
Toxicol Lett. 2012 Dec 17;215(3):174-80.
Osteosarcoma is one of the most common malignant bone tumors in children and adolescents. Although extensive efforts have been made in anti-osteosarcoma therapy in recent decades, there are no effective low-toxicity drugs for treating patients with metastatic osteosarcoma. Hence, potent anti-metastatic osteosarcoma drugs are highly desired. In this study, we explored novel small molecular anti-metastatic osteosarcoma agents and found that Columbamine (COL), an active component of the herb Coptis chinensis, inhibited the proliferation and neovascularization of metastatic osteosarcoma U2OS cells. COL effectively suppressed U2OS cell proliferation in vitro with an IC(50) of 21.31+/-0.38muM, with low cytotoxicity. Mechanistic studies revealed that COL induces cell cycle arrest at the G2/M transition, which is associated with attenuating CDK6 gene expression and diminishing STAT3 phosphorylation. COL did not significantly promote U2OS cell apoptosis at any of the dosages tested. Additionally, COL inhibited U2OS cell-mediated neovascularization, which was accompanied by the down-regulation of matrix metalloproteinase (MMP) 2 expression and reduction of cell migration, adhesion, and invasion. Taken together, our data show that COL exerts anti-proliferative and anti-vasculogenic effects on metastatic human osteosarcoma U2OS cells with low toxicity. These results warrant further investigation of COL as a potential anti-osteosarcoma and anti-cancer drug.
Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa.[Pubmed:24448061]
Molecules. 2014 Jan 20;19(1):1201-11.
Quaternary alkaloids are the major alkaloids isolated from Tinospora species. A previous study pointed to the necessary presence of quaternary nitrogens for strong acetylcholinesterase (AChE) inhibitory activity in such alkaloids. Repeated column chromatography of the vine of Tinospora crispa extract led to the isolation of one new protoberberine alkaloid, 4,13-dihydroxy-2,8,9-trimethoxydibenzo[a,g]quinolizinium (1), along with six known alkaloids-dihydrodiscretamine (2), Columbamine (3), magnoflorine (4), N-formylannonaine (5), N-formylnornuciferine (6), and N-trans-feruloyltyramine (7). The seven compounds were isolated and structurally elucidated by spectroscopic analysis. Two known alkaloids, namely, dihydrodiscretamine and Columbamine are reported for the first time for this plant. The compounds were tested for AChE inhibitory activity using Ellman's method. In the AChE inhibition assay, only Columbamine (3) showed strong activity with IC50 48.1 microM. The structure-activity relationships derived from these results suggest that the quaternary nitrogen in the skeleton has some effect, but that a high degree of methoxylation is more important for acetylcholinesterase inhibition.


