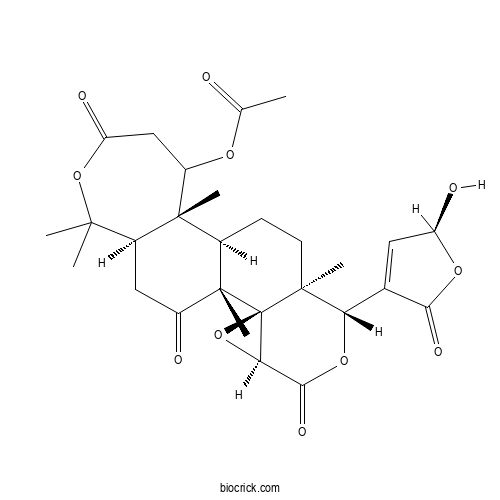
CitrusinCAS# 108943-57-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108943-57-3 | SDF | Download SDF |
| PubChem ID | 101127372 | Appearance | Powder |
| Formula | C28H34O11 | M.Wt | 546.6 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R,2R,4S,7R,8S,11R,12R,18R)-7-[(2S)-2-hydroxy-5-oxo-2H-furan-4-yl]-1,8,12,17,17-pentamethyl-5,15,20-trioxo-3,6,16-trioxapentacyclo[9.9.0.02,4.02,8.012,18]icosan-13-yl] acetate | ||
| SMILES | CC(=O)OC1CC(=O)OC(C2C1(C3CCC4(C(OC(=O)C5C4(C3(C(=O)C2)C)O5)C6=CC(OC6=O)O)C)C)(C)C | ||
| Standard InChIKey | ZYTRZCSXUOZYBK-OSQFVGJVSA-N | ||
| Standard InChI | InChI=1S/C28H34O11/c1-12(29)35-17-11-19(32)38-24(2,3)15-10-16(30)27(6)14(26(15,17)5)7-8-25(4)20(13-9-18(31)36-22(13)33)37-23(34)21-28(25,27)39-21/h9,14-15,17-18,20-21,31H,7-8,10-11H2,1-6H3/t14-,15+,17?,18+,20+,21-,25+,26-,27+,28-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Citrusin Dilution Calculator

Citrusin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8295 mL | 9.1475 mL | 18.2949 mL | 36.5898 mL | 45.7373 mL |
| 5 mM | 0.3659 mL | 1.8295 mL | 3.659 mL | 7.318 mL | 9.1475 mL |
| 10 mM | 0.1829 mL | 0.9147 mL | 1.8295 mL | 3.659 mL | 4.5737 mL |
| 50 mM | 0.0366 mL | 0.1829 mL | 0.3659 mL | 0.7318 mL | 0.9147 mL |
| 100 mM | 0.0183 mL | 0.0915 mL | 0.1829 mL | 0.3659 mL | 0.4574 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (12Z)-Labda-8(17),12-diene-14,15,16-triol
Catalog No.:BCX0441
CAS No.:1630864-26-4
- Thalifaramine
Catalog No.:BCX0440
CAS No.:105437-16-9
- Huazhongilexol
Catalog No.:BCX0439
CAS No.:161407-80-3
- Thalifaronine
Catalog No.:BCX0438
CAS No.:105458-70-6
- 1(10)Z,4Z-Furanodienone
Catalog No.:BCX0437
CAS No.:88010-63-3
- 5-Deoxyisorhoifolin
Catalog No.:BCX0436
CAS No.:2055239-29-5
- Anibadimer A
Catalog No.:BCX0435
CAS No.:23768-65-2
- Isolimonic acid
Catalog No.:BCX0434
CAS No.:74729-97-8
- (2Z,6E)-Farnesyl acetate
Catalog No.:BCX0433
CAS No.:40266-29-3
- Carbendazim
Catalog No.:BCX0432
CAS No.:10605-21-7
- Anisyl alcohol
Catalog No.:BCX0431
CAS No.:105-13-5
- Deacetylnomilinic acid
Catalog No.:BCX0430
CAS No.:35930-21-3
- 7-O-Galloyl-D-sedoheptulose
Catalog No.:BCX0443
CAS No.:233690-85-2
- 1,7-Di-O-Galloyl-D-sedoheptulose
Catalog No.:BCX0444
CAS No.:126622-78-4
- Durianol A
Catalog No.:BCX0445
CAS No.:1941203-91-3
- Morofficinaloside
Catalog No.:BCX0446
CAS No.:78964-45-1
- 14-Deoxyisocoronarin D
Catalog No.:BCX0447
CAS No.:189239-51-8
- Zerumin
Catalog No.:BCX0448
CAS No.:180680-70-0
- 7,4'-Dihydroxyflavone 7-O-glucoside
Catalog No.:BCX0449
CAS No.:20633-86-7
- (2E,4E)-6-Hydroxy-2,6-dimethylhepta-2,4-dienal
Catalog No.:BCX0450
CAS No.:220766-63-2
- Isoscutellarein 8-O-glucuronide
Catalog No.:BCX0451
CAS No.:56317-09-0
- Zerumin B
Catalog No.:BCX0452
CAS No.:176050-49-0
- 6'-O-Glucopyranosylpaeoniflorin
Catalog No.:BCX0453
CAS No.:1427054-21-4
- 21,23-Dihydro-21-hydroxy-23-oxonomilinic acid methyl ester
Catalog No.:BCX0454
CAS No.:2243600-34-0
Chaenomelin, a New Phenolic Glycoside, and Anti-Helicobacter pylori Phenolic Compounds from the Leaves of Salix chaenomeloides.[Pubmed:38475547]
Plants (Basel). 2024 Feb 29;13(5):701.
Salix chaenomeloides Kimura, commonly known as pussy willow, is a deciduous shrub and tree belonging to the Salicaceae family. The genus Salix spp. has been known as a healing herb for the treatment of fever, inflammation, and pain relief. The current study aimed to investigate the potential bioactive natural products from S. chaenomeloides leaves and evaluate their antibacterial activity against Helicobacter pylori. A phytochemical investigation of the ethanol (EtOH) extract of S. chaenomeloides leaves led to the isolation of 13 phenolic compounds (1-13) from the ethyl acetate (EtOAc) fraction, which showed antibacterial activity against H. pylori strain 51. The chemical structure of a new phenolic glycoside, chaenomelin (1), was established by a detailed analysis of 1D and 2D ((1)H-(1)H correlation spectroscopy (COSY), heteronuclear single-quantum coherence (HSQC), and heteronuclear multiple-bond correlation (HMBC)) nuclear magnetic resonance (NMR), high-resolution electrospray ionization mass spectroscopy (HR-ESIMS), and chemical reactions. The other known compounds were identified as 5-O-trans-p-coumaroyl quinic acid methyl ester (2), tremulacin (3), Citrusin C (4), benzyl 3-O-beta-d-glucopyranosyl-7-hydroxybenzoate (5), tremuloidin (6), 1-[O-beta-d-glucopyranosyl(1-->2)-beta-d-glucopyranosyl]oxy-2-phenol (7), arbutin cinnamate (8), tremulacinol (9), catechol (10), 4-hydroxybenzaldehyde (11), kaempferol 3-rutinoside (12), and narcissin (13), based on the comparison of their NMR spectra with the reported data and liquid chromatography/mass spectrometry (LC/MS) analysis. The isolated compounds were evaluated for antibacterial activity against H. pylori strain 51. Among the isolates, 1-[O-beta-d-glucopyranosyl(1-->2)-beta-d-glucopyranosyl]oxy-2-phenol (7) and arbutin cinnamate (8) exhibited antibacterial activity against H. pylori strain 51, with inhibitions of 31.4% and 33.9%, respectively, at a final concentration of 100 muM. These results were comparable to that of quercetin (38.4% inhibition), which served as a positive control. Generally, these findings highlight the potential of the active compounds 7 and 8 as antibacterial agents against H. pylori.
Flavonoids contribute most to discriminating aged Guang Chenpi (Citrus reticulata 'Chachi') by spectrum-effect relationship analysis between LC-Q-Orbitrap/MS fingerprint and ameliorating spleen deficiency activity.[Pubmed:37970411]
Food Sci Nutr. 2023 Sep 21;11(11):7039-7060.
To further explore the mechanism of "the longer storage time, the better bioactivity" of aged Guang Chenpi, the dry pericarp of Citrus reticulata 'Chachi' (CRC), a series of activity assessments were performed on spleen deficiency mice. The constituents in CRC with different storage years were analyzed by LC-Q-Orbitrap/MS. A total of 53 compounds were identified, and CRC stored for more than 5 years showed higher flavonoid content, especially that of polymethoxyflavones. Anti-spleen deficiency bioactivity analysis among various CRC with different storage years showed aged CRC (stored for more than 3 years) could significantly alleviate fatigue and depression behaviors much better, increase D-xylose and gastrin secretion, and upregulate the expression of the linking protein occludin in the colon walls. Results from 16S rDNA sequencing showed that aged CRC could downregulate the abundance of Enterococcus, Gemmata, Citrobacter, Escherichia_Shigella, and Klebsiella, which were significantly overrepresented in the model group. Bacteroides, Muribaculum, Alloprevotella, Paraprevotella, Alistipes, Eisenbergiella, and Colidextribacter were downregulated in the model group but enriched in the CRC groups. At last, the spectrum-effect relationship analysis indicated that flavonoids such as Citrusin III, homoeriodictyol, hesperidin, nobiletin, and isosinensetin in aged CRC showed the highest correlation with better activity in ameliorating spleen deficiency by regulating gut microbiota. Flavonoids contribute most to discriminating aged CRC and could disclose the basis of "the longer storage time, the better bioactivity" of aged Guang Chenpi.
Updates on RPE cell damage in diabetic retinopathy (Review).[Pubmed:37594078]
Mol Med Rep. 2023 Oct;28(4):185.
Diabetic retinopathy (DR) is a microvascular complication of diabetes. The retinal pigment epithelium (RPE) forms the outer layer of the blood‑retinal barrier and serves a role in maintaining retinal function. RPE cell injury has been revealed in diabetic animal models, and high glucose (HG) levels may cause damage to RPE cells by increasing the levels of oxidative stress, promoting pro‑inflammatory gene expression, disrupting cell proliferation, inducing the endothelial‑mesenchymal transition, weakening tight conjunctions and elevating cell death mechanisms, such as apoptosis, ferroptosis and pyroptosis. Non‑coding RNAs including microRNAs, long non‑coding RNAs and circular RNAs participate in RPE cell damage caused by HG levels, which may provide targeted therapeutic strategies for the treatment of DR. Plant extracts such as Citrusin and hesperidin, and a number of hypoglycemic drugs, such as sodium‑glucose co‑transporter 2 inhibitors, metformin and glucagon‑like peptide‑1 receptor agonists, exhibit potential RPE protective effects; however, the detailed mechanisms behind these effects remain to be fully elucidated. An in‑depth understanding of the contribution of the RPE to DR may provide novel perspectives and therapeutic targets for DR.
Phytochemical constituents from Elsholtzia ciliata (Thunb.) Hyl. and their nitric oxide production inhibitory activities.[Pubmed:36377760]
Nat Prod Res. 2023 Aug-Sep;37(18):3093-3102.
A new megastigmane glycoside, (3S,4R,7E)-megastigma-5,7-diene-9-one-3,4-diol 3-O-beta-D-apiofuranosyl-(1-->2)-beta-D-glucopyranoside (1) and a new cyanogenic glycosyl derivative, (S)-2-(6'-O-R-rosmarinoyl-beta-D-glucopyranosyloxy)-phenylacetonitrile (2) were isolated from the methanol extract of the Elsholtzia ciliata together with twelve known compounds, 1-O-beta-D-glucopyranosyl-2-hydroxy-4-allylbenzene (3), Citrusin C (4), 1,2-di-O-beta-D-glucopyranosyl-4-allylbenzene (5), manglieside B (6), 4-allyl-2-hydroxyphenyl 1-O-beta-D-apiofuranosyl-(1-->6)-beta-D-glucopyranoside (7), (-)-isolariciresinol 3alpha-beta-D-glucopyranoside (8), 7R,8R-threo-4,7,9-trihydroxy-3,3'-dimethoxy-8-O-4'-neolignan-9'-O-beta-D-glucopyranoside (9), 7R,8R-threo-4,7,9,9'-tetrahydroxy-3-methoxy-8-O-4'-neolignan-9'-O-beta-D-glucopyranoside (10), cedrusin-4-O-beta-D-glucopyranoside (11), icariside E(3) (12), everlastoside L (13) and rosmarinic acid (14). Their chemical structures were elucidated on the basic of extensive 1D and 2D-NMR experiments, as well as their mass spectroscopic data. The absolute configurations of the compounds 1 and 2 were successfully indicated by both theoretical and calculated CD spectra. Compounds 3-7, 9 and 10 potential inhibited NO production in LPS-activated RAW264.7 cells with IC(50) values of 6.71, 8.97, 12.38, 14.27, 16.13, 13.54, 16.27 microM, respectively, compared to that of the positive control of N(G)-monomethyl-L-arginine acetate (L-NMMA), IC(50) = 32.51 microM.
Constituents of Tinospora sinensis and their nitric oxide inhibitory activities.[Pubmed:36069750]
J Asian Nat Prod Res. 2023 Jun;25(6):603-609.
One new phenylpropanoid glycoside, tinosinen A (1) and 13 known compounds, tinosinen (2), Citrusin B (3), picraquassioside C (4), erythro-guaiacylglycerol-beta-O-4'-coniferyl alcohol (5), erythro-guaiacylglycerol-8-O-4'-(sinapyl alcohol) ether (6), erythro-syringylglycerol-8-O-4'-(sinapyl alcohol) ether (7), seco-isolariciresinol 9-O-D-beta-glucopyranoside (8), tinosposide A (9), pinoresinol-4'-O-beta-D-glucopyranoside (10), syringaresinol-4'-O-beta-D-glucopyranoside (11), pinoresinol (12), syringaresinol (13), and lirioresino-beta-dimethyl ether (14) were isolated from the stems of Tinospora sinensis (Lour.) Merr. Their structures were established by detailed spectroscopic studies and comparisons with those reported in the literature. Compound 13 showed significant inhibitory NO production (IC(50) value of 38.53 +/- 1.90 muM) in RAW264.7 macrophages, LPS-stimulated. Compounds 3-7, 11, 12, and 14 inhibited NO production with IC(50) values ranging from 38.53 to 99.07 muM.
Investigation of effective natural inhibitors for starch hydrolysing enzymes from Simaroubaceae plants by molecular docking analysis and comparison with in-vitro studies.[Pubmed:35600433]
Heliyon. 2022 May 2;8(5):e09360.
The present study aims to find the effective natural enzyme inhibitors against alpha-amylase and alpha-glucosidase from the array of compounds identified in plants of the Simaroubaceae family using molecular docking and ADME/Toxicity studies. Among the 218 compounds docked against seven enzymes, buddlenol-A and Citrusin-B showed the best binding energies (kcal/mol) of -7.830 and -7.383 against human salivary alpha-amylase and pancreatic alpha-amylase respectively. The other two compounds 9-hydroxycanthin-6-one and bruceolline-B had the best binding energy of -6.461 and -7.576 against N-terminal and C-terminal maltase glucoamylase respectively. Whereas the binding energy of prosopine (-6.499) and fisetinidol (-7.575) was considered as the best against N-terminal and C-terminal sucrase-isomaltase respectively. Picrasidine-X showed the best binding energy (-7.592) against yeast alpha-glucosidase. The study revealed that the seven compounds which showed the best binding energy against respective enzymes are considered as the 'lead hit compounds'. Even though the 'lead hit compounds' are not obeying all the laws of ADMET, the drug-likeness properties of 9-hydroxycanthin-6-one, fisetinidol, picrasidine-X, and prosopine were considerable. Also, kaempferol-3-O-pentoside was the recent compound identified from the Simarouba glauca plant extract found to be one among the top five lead hit compounds against four enzymes. This study provides valuable insight into the direction of developing natural compounds as potential starch hydrolysing enzyme inhibitors for managing type 2 diabetes.
[Research on chemical constituents from Artemisia annua Ⅰ].[Pubmed:33787111]
Zhongguo Zhong Yao Za Zhi. 2021 Mar;46(5):1160-1167.
Chemical constituents were isolated and purified from the water extract of Artemisia annua by column chromatography of HP-20 macroporous resin, silica gel, ODS, Sephadex LH-20, HW-40, and semi-preparative RP-HPLC. Their structures were elucidated by physicochemical properties and spectral analyses. As a result, Fifteen compounds were isolated and identified as vitexnegheteroin M(1), sibricose A5(2), securoside A(3), Citrusin D(4), annphenone(5), E-melilotoside(6), esculetin(7), scopoletin-7-O-beta-D-glucoside(8), eleutheroside B_1(9), chrysosplenol D(10), patuletin-3-O-beta-D-glucopyranoside(11), quercetin-7-O-beta-D-glucoside(12), rutin(13), apigenin 6,8-di-C-beta-D-glucopyranoside(14), isoschaftoside(15), among them, compounds 1-4 were identified from Artemisia for the first time. Additionally, the isolates were evaluated for their inhibitory effects on the production of PGE_2 in LPS-simulated RAW264.7 macrophages. The results showed that compounds 1, 2, 8, and 10-15 could reduce PGE_2 levels, to a certain extent.
Human Saliva-Mediated Hydrolysis of Eugenyl-beta-D-Glucoside and Fluorescein-di-beta-D-Glucoside in In Vivo and In Vitro Models.[Pubmed:33514072]
Biomolecules. 2021 Jan 27;11(2):172.
Eugenyl-beta-D-glucopyranoside, also referred to as Citrusin C, is a natural glucoside found among others in cloves, basil and cinnamon plants. Eugenol in a form of free aglycone is used in perfumeries, flavourings, essential oils and in medicinal products. Synthetic Citrusin C was incubated with human saliva in several in vitro models together with substrate-specific enzyme and antibiotics (clindamycin, ciprofloxacin, amoxicillin trihydrate and potassium clavulanate). Citrusin C was detected using liquid chromatography with tandem mass spectrometry (LC-MS/MS). Citrusin C was completely degraded only when incubated with substrate-specific A. niger glucosidase E.C 3.2.1.21 (control sample) and when incubated with human saliva (tested sample). The addition of antibiotics to the above-described experimental setting, stopped Citrusin C degradation, indicating microbiologic origin of hydrolysis observed. Our results demonstrate that Citrusin C is subjected to complete degradation by salivary/oral cavity microorganisms. Extrapolation of our results allows to state that in the human oral cavity, virtually all beta-D-glucosides would follow this type of hydrolysis. Additionally, a new method was developed for an in vivo rapid test of glucosidase activity in the human mouth on the tongue using fluorescein-di-beta-D-glucoside as substrate. The results presented in this study serve as a proof of concept for the hypothesis that microbial hydrolysis path of beta-D-glucosides begins immediately in the human mouth and releases the aglycone directly into the gastrointestinal tract.
Identification of Novel Human Serum Albumin (SA) Inhibitors from Scoparia Dulsis for Urolithiasis.[Pubmed:31393255]
Curr Comput Aided Drug Des. 2020;16(3):308-317.
BACKGROUND: Urolithiasis is the process of forming stones in the kidney, bladder, and/or urinary tract. It has been reported that kidney stones are the third most common disorder among urinary diseases. At present, surgical procedures and Extracorporeal Shock Wave Lithotripsy (ESWL) are commonly employed for the treatment of Urolithiasis. The major drawback of these procedures is the recurrence of stones. METHODS: This study aimed to identify potential natural inhibitors against human Serum Albumin (SA) from the plant Scoparia Dulsis for Urolithiasis. As protein-ligand interactions play a key role in structure- based drug design, this study screened 26 compounds from Scoparia Dulsis and investigated their binding affinity against SA by using molecular docking. The three dimensional (3D) structure of SA was retrieved from Protein Data Bank (PDB) and docked with PubChem structures of 26 compounds using PyRX docking tool through Autodock Vina. Moreover, a 3D similarity search on the PubChem database was performed to find the analogs of best scored compound and docking studies were performed. Drug-likeness studies were made using Swiss ADME and Lipinski's rule of five was performed for the compounds to evaluate their anti-urolithiatic activity. RESULTS: The results showed that Citrusin c (Eugenyl beta-D-glucopyranoside) exhibited best binding energy of -8.1 kcal/mol with SA followed by aphidicolin, apigenin, luteolin and scutellarein. Two compounds (PubChem CID 46186820, PubChem CID 21579141) analogous to Citrusin c were selected based on the lowest binding energy. CONCLUSION: This study, therefore, reveals that these compounds could be promising candidates for further evaluation for Urolithiasis prevention or management.
A new C-glycosyl flavone and a new neolignan glycoside from Passiflora edulis Sims peel.[Pubmed:29199463]
Nat Prod Res. 2018 Oct;32(19):2312-2318.
A new C-glycosyl flavone, Chrysin-8-C-(2''-O-beta-6-deoxy-glucopyranosyl)-beta-D-glucopyranoside (1), a new neolignan glycoside, Citrusin G (2), as well as 15 known compounds (3-17) were isolated from the peel of Passiflora edulis Sims. The structure determinations were primarily based on comprehensive spectroscopic analyses, and the absolute configuration of 2 were unequivocally determined by the CD experiment and chemical transformation. Compound 1 represents the rare examples of the flavonoid featuring a deoxy glucose sugar moiety. Compounds 5, 7 and 9 exhibited moderate inhibitory effects on nitric oxide (NO) production stimulated by lipopolysaccharide (LPS) in RAW 264.7 cells, with IC(50) values of 34.92, 16.12 and 26.67 muM, respectively.
A new sesquiterpene from Kalimeris integrifolia.[Pubmed:28927288]
Nat Prod Res. 2018 May;32(9):1004-1009.
A new sesquiterpene kalinturoside A (1), and 17 known compounds friedelan-3-ol (2), 24-ethyl-5a-cholesta-7, 22(E)-dien-3-one (3), friedelin (4), syringaresinol (5), alpha-spinasterol (6), ciwujiatone (7), syringic acid (8), scopoletin (9), apocynin (10), 1-(3-hydroxy-4, 5-dimethoxyphenyl)ethan-1-one (11), apigenin (12), 5-hydroxymethylfurfural (13), stigmasterol-3-O-beta-d-glucopy-ranoside (14), bidenoside C (15), Citrusin (16), irioresinol A (17) and syringaresinol-4-O-beta-d-glucopyranoside (18) were isolated from the herbs of Kalimeris integrifolia. The structures of these compounds were elucidated using spectroscopic techniques such as NMR and MS. All of the compounds were isolated from this genus for the first time.
[Lignans from stems of Cistanche deserticola cultured in Tarim desert].[Pubmed:26084171]
Zhongguo Zhong Yao Za Zhi. 2015 Feb;40(3):463-8.
In order to clarify the chemical constituents of Cistanche deserticola cultured in Tarim desert, a systematically phytochemical investigation was carried out. The chemical constituents were isolated by column chromatography, such as silica gel, Sephadex LH- 20, MCI gel, ODS and semi-preparative HPLC, and their structures were determined on the basis of MS, NMR spectroscopic analysis and/or comparison with literature data. Eleven lignans were isolated from the 85% ethanol extract of the stems of C. deserticola cultured in Tarim desert. Their structures were identified as (+)-syringaresinol-4'-O-beta-D-glucopyranoside (1), (+)-isoeucommin A (2), eucommin A (3), (+)-pinoresinol monomethylether beta-D-glucoside (4), lariciresinol 4'-O-beta-D-glucopyranoside (5), lariciresinol 4-O-beta-D-glucopyranoside (6), conicaoside (7), dehydrodiconiferyl alcohol 4-O-beta-D-glucopyranoside (8), dehydrodiconiferyl alcohol gamma'-O-beta-D-glucoside (9), Citrusin A (10), and alaschanioside A (11). Compounds 1, 3-7, 10 and 11 were isolated from this genus for the first time, and compounds 2, 8 and 9 were obtained from this species for the first time.
Anti-inflammatory activity of a new cyclic peptide, citrusin XI, isolated from the fruits of Citrus unshiu.[Pubmed:25625351]
J Ethnopharmacol. 2015 Apr 2;163:106-12.
ETHNOPHARMACOLOGICAL RELEVANCE: Citrus unshiu (Rutaceae) is an easy-peeling citrus fruit, which has been used as a traditional Korean medicine for improving skin elasticity, relieving fatigue and cough, and preventing bronchitis, flu, and various cancers. However, its active components associated with anti-inflammation and underlying mechanisms remain unknown. In this study, we investigated the active constituents from the fruits of Citrus unshiu and evaluated the anti-inflammatory activity in order to support the traditional usage of Citrus unshiu. MATERIAL AND METHODS: Repeated column chromatography, together with a semi-preparative HPLC purification was used to separate the bioactive constituent from the EtOAc soluble fraction of the EtOH extract of Citrus unshiu fruits. Anti-inflammatory effects of the isolated compounds on lipopolysaccharide (LPS)-induced production of pro-inflammatory mediators were examined using RAW264.7 macrophage cells. RESULTS: A new cyclic peptide, Citrusin XI (1), was isolated and identified from the fruits of Citrus unshiu. The structure of compound 1 was elucidated by spectroscopic analysis, including 1D and 2D nuclear magnetic resonance (NMR) ((1)H, (13)C, COSY, HMQC and HMBC experiments), and high resolution (HR)-mass spectrometry, and its absolute configurations were further confirmed by the Marfey׳s method. Compound 1 decreased NO production in LPS-stimulated RAW264.7 cells in a dose-dependent manner with an IC50 value of 70muM. Compound 1 suppressed NO production by decreasing iNOS expression but COX-2 expression was slightly associated with the reduction by compound 1 in LPS-induced RAW264.7 cells. Furthermore, compound 1 inhibited NF-kappaB activation by blocking IkappaBalpha degradation and NF-kappaB phosphorylation in LPS-stimulated RAW264.7 cells. CONCLUSIONS: These results indicate that a new cyclic peptide, Citrusin XI, from Citrus unshiu fruits has anti-inflammatory properties that inhibit the release of pro-inflammatory mediators. Compound 1 decreases NO production by decreasing iNOS expression and NF-kappaB activation associated with IkappaBalpha degradation and NF-kappaB phosphorylation in LPS-induced RAW264.7 cells. This is the first study to clarify the underlying mechanism of the anti-inflammatory effect exerted by a pure isolated compound from Citrus unshiu in LPS-stimulated RAW264.7 macrophage cells. The phytochemical, Citrusin XI of Citrus unshiu may serve as lead compound in the design of new agents for preventing and treating inflammatory diseases.
[Study on lignans of Gentianella acuta].[Pubmed:25335286]
Zhong Yao Cai. 2014 May;37(5):800-3.
OBJECTIVE: To study the lignans from the whole plant of Gentianella acuta. METHODS: The compounds were isolated by various chromatographic techniques and indentified by spectroscopic methods. RESULTS: Nine compounds were isolated and identified as (7R,8S) -dehydrodiconiferyl alcohol-4,9'-O-beta-D-glucopyranoside (1), alaschanisoside A (2), Citrusin A (3), olivil-4'-O-beta-D-glucopyranoside (4), leptolepisol D (5), acanthoside D (6), (+) pinoresinol-4-O-beta-D-glucopyranoside (7), (+) 8-hydroxypinoresinol-4-O-beta-D-glucopyranoside (8), and (+) pinoresinol-8-O-beta-D-glucopyranoside (9). CONCLUSION: Compounds 1 - 9 are isolated from this plant for the first time, compounds 1 and 9 are isolated from Gentianella genus for the first time,and compounds 2, 3 and 5 - 8 are isolated from Gentianaceae family for the first time.
Metabolomic approaches for orange origin discrimination by ultra-high performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry.[Pubmed:24679755]
Food Chem. 2014 Aug 15;157:84-93.
In this work, hybrid quadrupole time-of-flight mass spectrometer (QTOF MS) coupled to ultra high performance liquid chromatography (UHPLC) has been used for biomarkers identification for correct authentication of Valencia (Spain) oranges. Differentiation from foreign Argentinean, Brazilian and South African oranges has been carried out using XCMS application and multivariate analysis to UHPLC-(Q)TOF MS data acquired in both, positive and negative ionisation modes. Several markers have been found and corroborated by analysing two seasons samples. A seasonal independent marker was found and its structure elucidated using accurate mass data and MS(E) fragmentation spectrum information. Empirical formula was searched in Reaxys database applying sub-structure filtering from the fragments obtained. Three possible structures were found and Citrusin D, a compound present in sweet oranges, has been identified as the most plausible as it fits better with the product ion scan performed for this compound. As a result of data obtained in this work, Citrusin D is suggested as a potential marker to distinguish the geographic origin of oranges.


