Citroside ACAS# 120330-44-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

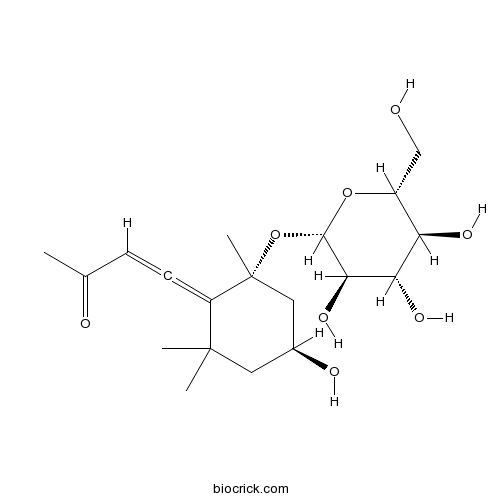
| Cas No. | 120330-44-1 | SDF | Download SDF |
| PubChem ID | 14312562 | Appearance | Powder |
| Formula | C19H30O8 | M.Wt | 386.44 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC(=O)C=C=C1C(CC(CC1(C)OC2C(C(C(C(O2)CO)O)O)O)O)(C)C | ||
| Standard InChIKey | XTODSGVDHGMKSN-SIEIHWOKSA-N | ||
| Standard InChI | InChI=1S/C19H30O8/c1-10(21)5-6-13-18(2,3)7-11(22)8-19(13,4)27-17-16(25)15(24)14(23)12(9-20)26-17/h5,11-12,14-17,20,22-25H,7-9H2,1-4H3/t6?,11-,12+,14+,15-,16+,17-,19+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Citroside A is a natural product from Cirsium setosum. |
| In vitro | Study on active constituents against Alzheimer's disease from Valeriana amurensis.[Pubmed: 28891614]Zhongguo Zhong Yao Za Zhi. 2016 May;41(9):1649-1653.
|
| Structure Identification | Chin J Nat Med. 2013 Sep;11(5):534-7.A new megastigmane glycoside from the aerial parts of Cirsium setosum.[Pubmed: 24359780 ]To study the chemical constituents of the aerial parts of Cirsium setosum (Willd.) MB.. |

Citroside A Dilution Calculator

Citroside A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5877 mL | 12.9386 mL | 25.8772 mL | 51.7545 mL | 64.6931 mL |
| 5 mM | 0.5175 mL | 2.5877 mL | 5.1754 mL | 10.3509 mL | 12.9386 mL |
| 10 mM | 0.2588 mL | 1.2939 mL | 2.5877 mL | 5.1754 mL | 6.4693 mL |
| 50 mM | 0.0518 mL | 0.2588 mL | 0.5175 mL | 1.0351 mL | 1.2939 mL |
| 100 mM | 0.0259 mL | 0.1294 mL | 0.2588 mL | 0.5175 mL | 0.6469 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CX-6258
Catalog No.:BCC1504
CAS No.:1202916-90-2
- Dorzolamide
Catalog No.:BCC4287
CAS No.:120279-96-1
- 4-Hydroxysapriparaquinone
Catalog No.:BCN4806
CAS No.:120278-25-3
- Salvinolone
Catalog No.:BCN3215
CAS No.:120278-22-0
- CNX-774
Catalog No.:BCC4394
CAS No.:1202759-32-7
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- CGS 21680
Catalog No.:BCC1475
CAS No.:120225-54-9
- 1beta-Hydroxyeuscaphic acid
Catalog No.:BCN3517
CAS No.:120211-98-5
- Tenidap
Catalog No.:BCC7419
CAS No.:120210-48-2
- NMS-P715
Catalog No.:BCC6373
CAS No.:1202055-34-2
- Clopidogrel Related Compound C
Catalog No.:BCN2689
CAS No.:120202-71-3
- Clopidogrel
Catalog No.:BCC2497
CAS No.:120202-66-6
- DASA-58
Catalog No.:BCC6522
CAS No.:1203494-49-8
- Cyclotraxin B
Catalog No.:BCC6357
CAS No.:1203586-72-4
- AS 1949490
Catalog No.:BCC7762
CAS No.:1203680-76-5
- Jionoside B1
Catalog No.:BCN2858
CAS No.:120406-37-3
- Biapenem
Catalog No.:BCC1071
CAS No.:120410-24-4
- AZD1208
Catalog No.:BCC2079
CAS No.:1204144-28-4
- Icotinib Hydrochloride
Catalog No.:BCC1639
CAS No.:1204313-51-8
- TMC647055
Catalog No.:BCC6376
CAS No.:1204416-97-6
- Verlukast
Catalog No.:BCC2035
CAS No.:120443-16-5
- Jionoside A1
Catalog No.:BCN2922
CAS No.:120444-60-2
- (±)-Vesamicol hydrochloride
Catalog No.:BCC6737
CAS No.:120447-62-3
- ER 27319 maleate
Catalog No.:BCC5914
CAS No.:1204480-26-1
Skin depigmenting action of silkworm (Bombyx mori L.) droppings in zebrafish.[Pubmed:29356892]
Arch Dermatol Res. 2018 Apr;310(3):245-253.
The excrement of silkworms (Bombyx mori L.), referred to here as silkworm droppings (SDs), is used as a traditional drug in eastern medicine to treat skin diseases such as urticaria and atopy. However, the depigmentation effects of SDs have not previously been evaluated. We focused on the depigmentation effect of a methanol extract of SDs and isolated components of the extract using a zebrafish model system. (+)-Dehydrovomifoliol (M-1), (6R,7E,9R)-9-hydroxy-4,7-megastigmadien-3-one (M-2), (3S,5R,8R)-3,5-dihydroxymegastigma-6,7-dien-9-one (M-3), roseoside (M-4), and Citroside A (M-5) were isolated from only SDs extract (SDE), and chemical structures were identified through spectroscopic methods. Toxicity of SDE was evaluated by assessing its effect on the viability of human fibroblast cells and the hatching rate of zebrafish embryos. In addition, the depigmentation ability of SDE and isolated constituents was evaluated using a zebrafish model. Binary threshold, histograms, and the size of the black spots on the dorsal region of zebrafish larvae were analyzed using image analysis tools. Finally, SDE is a non-toxic material and has a dose-dependent depigmentation effect in zebrafish larvae. Moreover, various doses of compounds isolated from SDE, namely, M-1 to M-5, had a depigmentation effect. In particular, M-5 inhibited melanin synthesis in melanocytes stimulated by alpha-melanocyte stimulating hormone (alpha-MSH). Together, our results suggest that SDs can be used for depigmentation purposes in health and/or cosmetic applications.
Crotonionosides A-G: megastigmane glycosides from leaves of Croton cascarilloides Rauschel.[Pubmed:21055781]
Phytochemistry. 2011 Jan;72(1):147-53.
From the 1-BuOH-soluble fraction of a MeOH extract of leaves of Croton cascarilloides, collected in Okinawa, Japan, seven megastigmane glycosides, named crotonionosides A-G, were isolated together with three known megastigmane glucosides, dendranthemosides A and B, and Citroside A. This structures were elucidated by a combination of spectroscopic analyses, HPLC analyses, and application of the modified Mosher's method.
Schefflerins A-G, new triterpene glucosides from the leaves of Schefflera arboricola.[Pubmed:20930402]
Chem Pharm Bull (Tokyo). 2010 Oct;58(10):1343-8.
From the 1-BuOH-soluble fraction of a MeOH extract of leaves of Schefflera arboricola, collected in Okinawa, six new lupane glucosides, named schefflerins A-F (1-6) and one new dammarane glucoside, named schefflerin G (7), were isolated together with three known compounds, Citroside A (8), and oleanane saponins, oleanolic acid (9) and echinocystic acid (10) 3-O-alpha-L-rhamnopyranosyl(1-->4')-O-beta-D-glucuronopynosides. Their structures were elucidated through a combination of spectroscopic analyses and the structure of schefflerin F (6) was determined by X-ray crystallographic method using SPring-8 synchrotron radiation.
Cuneatoside, a new megastigmane diglycoside from Erythroxylum cuneatum Blume.[Pubmed:17145665]
J Asian Nat Prod Res. 2006 Dec;8(8):747-51.
A new megastigmane diglycoside, inamoside 6'-O-L-alpha-arabinofuranoside (cuneatoside), was isolated from the leaves and branches of Erythroxylum cuneatum together with seven known compounds, (+)-catechin, quercetin 3-O-alpha-L-rhamnoside, apocynol B, (6S,9R)-roseoside, vomifoliol 9-O-alpha-L-arabinofuranosyl (1-->6)-beta-D-glucopyranoside, inamoside, and Citroside A The structural elucidations were based on analyses of physical and spectroscopic data.
[Study on active constituents against Alzheimer's disease from Valeriana amurensis].[Pubmed:28891614]
Zhongguo Zhong Yao Za Zhi. 2016 May;41(9):1649-1653.
In this study, the chemical constituentsfrom Valeriana amurensis AD-effective fraction were investigated based on the effect of Valeriana amurensis on Alzheimer's disease (AD) in previous study. Valeriana amurensis was extracted with 75% ethanol and the obtained extract were extracted and subjected to AB-8 macroporous resin column to obtain the AD-effective fraction of Valeriana amurensis. 9 compounds (1-9) were isolated with silica gel, ODS column chromatography and preparative HPLC. The structures of these compounds were determined as 6-hydroxy-7-(hydroxymethyl)-4-methylenehexahydrocyclopenta[c]-pyran-1(3H)-one (1), suspensolide F (2), loganin(3), alpha-morroniside(4), beta-morronisid (5), partinovalerosidate (6), zansiumloside A (7), (-)-angelicoidenol-2-O-beta-D-glucopyranoside (8), Citroside A (9). Compounds 6-9 were isolated from the valerian genus for the first time and further investigated the anti-AD effect of compounds 1-9 in vitro found that compound 2 and 6 protected the PC12 cells from injury significantly.
Two new megastigmane glycosides, physanosides A and B, from Physalis alkekengi L. var. franchetii, and their effect on NO release in macrophages.[Pubmed:18493962]
Chem Biodivers. 2008 May;5(5):758-63.
Two new megastigmane glycosides, physanosides A and B (1 and 2, resp.), were isolated from Physalis alkekengi L. var. franchetii, together with four known compounds (6S,9R)-roseoside (3), (6S,9S)-roseoside (4), (6R,9S)-3-oxo-alpha-ionol beta-D-glucopyranoside (5), and Citroside A (6). Their structures were elucidated on the basis of physicochemical evidence, in-depth NMR spectroscopic analysis, high-resolution mass spectrometry, and CD spectroscopy, and their inhibitory effect on NO production was also examined. Compounds 2 and 3 exhibited strong inhibition on lipopolysaccharide-induced NO release by macrophages with IC(50) values of 9.93 and 7.31 microM, respectively.
Elaeocarpionoside, a megastigmane glucoside from the leaves of Elaeocarpus japonicus Sieb. et Zucc.[Pubmed:20091243]
J Nat Med. 2010 Jan;64(1):104-8.
From a 1-BuOH-soluble fraction of an extract of the leaves of Elaeocarpus japonicus, six megastigmane glucosides (1-6) were isolated. Five were known compounds (2-6), i.e. amelopsisionoside, Citroside A, roseoside, alangionoside A and turpinionoside A, respectively. The structure of the new compound, named elaeocarpionoside, was elucidated to be (3R,4R,5S,6S,7E,9R)-megastigman-7-ene-3,4,9-triol 9-O-beta-D: -glucopyranoside by spectroscopic analyses and the modified Mosher's method.


