CichoriinCAS# 531-58-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 531-58-8 | SDF | Download SDF |
| PubChem ID | 442101 | Appearance | Powder |
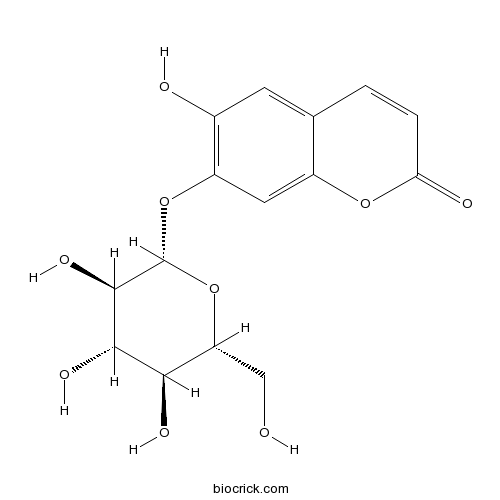
| Formula | C15H16O9 | M.Wt | 340.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-hydroxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-2-one | ||
| SMILES | C1=CC(=O)OC2=CC(=C(C=C21)O)OC3C(C(C(C(O3)CO)O)O)O | ||
| Standard InChIKey | WNBCMONIPIJTSB-TVKJYDDYSA-N | ||
| Standard InChI | InChI=1S/C15H16O9/c16-5-10-12(19)13(20)14(21)15(24-10)23-9-4-8-6(3-7(9)17)1-2-11(18)22-8/h1-4,10,12-17,19-21H,5H2/t10-,12-,13+,14-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cichoriin is a photosensitive compound, it could be used as herbal photosensitizing agent in treating benign breast tumor in rats. |
| In vitro | Cytoprotective Activity of Chemical Constituents Isolated from Streptomyces sp.[Reference: WebLink]International Journal of Biological Chemistry, 2009, 3(1):11-17.
|
| In vivo | Introducing Cichorium Pumilum as a potential therapeutical agent against drug-induced benign breast tumor in rats.[Pubmed: 22812448]Electromagn Biol Med. 2012 Dec;31(4):299-309.Cichorium Pumilum (chicory) is could be a promising cancer treatment in which a photosensitizing drug concentrates in benign tumor cells and activated by quanta at certain wavelength. Such activated extracts could lead to cell death and tumor ablation. Previous studies have shown that Cichorium Pumilum (chicory) contains photosensitive compounds such as
Cichoriin, anthocyanins, lactucin, and Lactucopicrin. |

Cichoriin Dilution Calculator

Cichoriin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9386 mL | 14.6929 mL | 29.3858 mL | 58.7717 mL | 73.4646 mL |
| 5 mM | 0.5877 mL | 2.9386 mL | 5.8772 mL | 11.7543 mL | 14.6929 mL |
| 10 mM | 0.2939 mL | 1.4693 mL | 2.9386 mL | 5.8772 mL | 7.3465 mL |
| 50 mM | 0.0588 mL | 0.2939 mL | 0.5877 mL | 1.1754 mL | 1.4693 mL |
| 100 mM | 0.0294 mL | 0.1469 mL | 0.2939 mL | 0.5877 mL | 0.7346 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- [(1(10)E,2R,4R)]-2-Methoxy-8,12-epoxygemacra-1(10),7,11-trien-6-one
Catalog No.:BCN8899
CAS No.:75412-95-2
- 4,4-di(4-hydroxy-3-methoxyphenly)-2,3-dimethylbutanol
Catalog No.:BCN8898
CAS No.:913643-31-9
- 6'-Feruloylnodakenin
Catalog No.:BCN8897
CAS No.:131623-14-8
- N-Methylcorydalmine
Catalog No.:BCN8896
CAS No.:81010-29-9
- Prionanthoside
Catalog No.:BCN8895
CAS No.:161842-81-5
- Quercetin 7-O-(6''-O-malonyl)-beta-D-glucoside
Catalog No.:BCN8894
CAS No.:98767-37-4
- Andrographidine A
Catalog No.:BCN8893
CAS No.:113963-37-4
- Epischisandrone
Catalog No.:BCN8892
CAS No.:98619-26-2
- 1,2-Epoxy-10(14)-furanogermacren-6-one
Catalog No.:BCN8891
CAS No.:383368-24-9
- Bruceantinol B
Catalog No.:BCN8890
CAS No.:1822332-33-1
- Notoptol
Catalog No.:BCN8889
CAS No.:88206-49-9
- 2-Methoxyfuranoguaia-9-ene-8-one
Catalog No.:BCN8888
CAS No.:88010-62-2
- Spinacetin
Catalog No.:BCN8901
CAS No.:3153-83-1
- (E)-6-O-(p-coumaroyl)scandoside methyl ester
Catalog No.:BCN8902
CAS No.:83946-90-1
- 3-Acetyl-ginsenoside F1
Catalog No.:BCN8903
CAS No.:1881225-08-6
- Nortrachelogenin-8'-O-beta-glucoside
Catalog No.:BCN8904
CAS No.:858127-38-5
- Stevioside D
Catalog No.:BCN8905
CAS No.:1310055-59-4
- Isolappaol C
Catalog No.:BCN8906
CAS No.:929905-15-7
- Arisanschinin E
Catalog No.:BCN8907
CAS No.:1333378-33-8
- Rebaudioside F
Catalog No.:BCN8908
CAS No.:438045-89-7
- Arisantetralone B
Catalog No.:BCN8909
CAS No.:1161947-96-1
- Biatractylolide
Catalog No.:BCN8910
CAS No.:182426-37-5
- Methyl neochebulinate
Catalog No.:BCN8911
CAS No.:1236310-34-1
- 2-Methoxy-5-acetoxy-furanogermacr-1(10)-en-6-one
Catalog No.:BCN8912
CAS No.:1809980-25-3
Chemical Constituents from Fraxinus hupehensis and Their Antifungal and Herbicidal Activities.[Pubmed:31906487]
Biomolecules. 2020 Jan 2;10(1). pii: biom10010074.
The phytochemical investigation of Fraxinus hupehensis led to the isolation and characterization of ten compounds which were identified as fraxin (1), fraxetin (2), esculetin (3), Cichoriin (4), euphorbetin (5), kaempferol-3-O-beta-rutinoside (6), oleuropein (7), linoleic acid (8), methyl linoleate (9), and beta-sitosterol (10). Structures of the isolated constituents were characterized by (1)H NMR, (13)C NMR and HRMS. All the compounds, except compounds 3 and 4, were isolated for the first time from this plant. Further, this was the first report for the occurrence of compound 5 in the Fraxinus species. Antifungal activity evaluation showed that compound 2 exhibited significant inhibitory effects against Bipolaris maydis, Sclerotium rolfsii, and Alternaria solani with EC50 values of 0.31 +/- 0.01 mmol/L, 10.50 +/- 0.02 mmol/L, and 0.40 +/- 0.02 mmol/L respectively, compared to the positive control, Carbendazim, with its EC50 values of 0.74 +/- 0.01 mmol/L, 1.78 +/- 0.01 mmol/L and 1.41 +/- 0.00 mmol/L. Herbicidal activity tests showed that compounds 8-10 had strong inhibitory effects against the roots of Echinochloa crus-galli with EC50 values of 1.16 +/- 0.23 mmol/L, 1.28 +/- 0.58 mmol/L and 1.33 +/- 0.35 mmol/L respectively, more potently active than that of the positive control, Cyanazine, with its EC50 values of 1.56 +/- 0.44 mmol/L. However, none of the compounds proved to be active against the tested bacteria (Erwinia carotovora, Pseudomonas syringae, and Ralstonia solanacearum).
Inhibitory activity of Podospermum canum and its active components on collagenase, elastase and hyaluronidase enzymes.[Pubmed:31614286]
Bioorg Chem. 2019 Dec;93:103330.
Present study is aimed to investigate in vitro inhibitory effects of the extract prepared from the aerial parts of Podospermum canum (syn: Scorzonera cana var. jacquiniana) (Asteraceae) on hyaluronidase, collagenase, and elastase enzymes using a bioassay-guided fractionation. Inhibitory effects of the extract, sub-extracts, fractions obtained by column chromatography, and isolated compounds on collagenase, elastase, and hyaluronidase were performed by using in vitro enzyme inhibitory assays based on spectrophotometric evaluation. The methanolic extract obtained from P. canum exhibited strong inhibitory activities on elastase and collagenase while the insignificant activity was observed on hyaluronidase. Through bioactivity-guided fractionation, the ethyl acetate and remaining water sub-extracts obtained from the methanolic extract displayed significant inhibitory activities on collagenase and elastase, while petroleum ether and chloroform extracts did not show any inhibitory activity. Eleven known compounds: arbutin, 6-O-caffeoylarbutin, Cichoriin, 3,5-dicaffeoylquinic acid methyl ester, apigenin 7-O-beta-glucoside, luteolin 7-O-beta-glucoside, apigenin 7-O-beta-rutinoside, isoorientin, orientin, vitexin, procatechuic acid, and new compound 4-hydroxy-benzoic acid 4-(6-O-alpha-rhamnopyranosyl-beta-glucopyranosyl) benzyl ester have been obtained from ethyl acetate sub-extract. Results of the present study have revealed that apigenin 7-O-beta-glucoside, luteolin 7-O-beta-glucoside, apigenin 7-O-beta-rutinoside, and isoorientin showed potent enzyme inhibitory activities. However, methanolic extract of P. canum displayed a greater inhibitory activity than fractions and isolated compounds both on collagenase and elastase.
Phytochemical Investigations on Chemical Constituents of Taraxacum bessarabicum (Hornem.) Hand.-Mazz. subsp. bessarabicum (Hornem.) Hand.-Mazz.[Pubmed:31089374]
Iran J Pharm Res. 2019 Winter;18(1):400-405.
Plants of the genus Taraxacum Wigg., have long been used as medicinal herbs. A phytochemical investigation of the aerial parts of Taraxacum bessarabicum (Hornem.) Hand.-Mazz. subsp. bessarabicum (Hornem.) Hand.-Mazz. (Asteraceae) yielded two coumarins [esculetin (1), Cichoriin (2)], three flavonoids [ luteolin (3), luteolin 7-O-beta-D-glucoside (4), gossypetin (5)] and six phenolic acids and their derivatives [ p-coumaric acid (6), caffeic acid (7), ferulic acid (8), chlorogenic acid methyl ester (9), 3,5-di-O-caffeoylquinic acid (10), 3,5-di-O-caffeoylquinic acid methyl ester (11)]. Their structures were established conclusively by UV, ESI-MS, 1-D and 2-D NMR spectra analyses and comparison with literature data. The presence of these compounds has been shown for the first time from this species. This is the first report of the isolation of compound 5 from the genus Taraxacum.
Ethnobotany of the genus Taraxacum-Phytochemicals and antimicrobial activity.[Pubmed:30039597]
Phytother Res. 2018 Nov;32(11):2131-2145.
Plants belonging to the genus Taraxacum have been used in traditional healthcare to treat infectious diseases including food-borne infections. This review aims to summarize the available information on Taraxacum spp., focusing on plant cultivation, ethnomedicinal uses, bioactive phytochemicals, and antimicrobial properties. Phytochemicals present in Taraxacum spp. include sesquiterpene lactones, such as taraxacin, mongolicumin B, and taraxinic acid derivatives; triterpenoids, such as taraxasterol, taraxerol, and officinatrione; and phenolic derivatives, such as hydroxycinnamic acids (chlorogenic, chicoric, and caffeoyltartaric acids), coumarins (aesculin and Cichoriin), lignans (mongolicumin A), and taraxacosides. Aqueous and organic extracts of different plant parts exhibit promising in vitro antimicrobial activity relevant for controlling fungi and Gram-positive and Gram-negative bacteria. Therefore, this genus represents a potential source of bioactive phytochemicals with broad-spectrum antimicrobial activity. However, so far, preclinical evidence for these activities has not been fully substantiated by clinical studies. Indeed, clinical evidence for the activity of Taraxacum bioactive compounds is still scant, at least for infectious diseases, and there is limited information on oral bioavailability, pharmacological activities, and safety of Taraxacum products in humans, though their traditional uses would suggest that these plants are safe.
Isolation of major phenolics from Launaea spinosa and their protective effect on HepG2 cells damaged with t-BHP.[Pubmed:26052623]
Pharm Biol. 2016;54(3):536-41.
CONTEXT: Some Launaea species (Asteraceae) are used traditionally to treat liver oxidative stress. OBJECTIVE: The present study investigates the protective effects of isolated compounds from Launaea spinosa Sch. Bip. (Asteraceae) against oxidative stress on t-BHP-induced HepG2 cells. MATERIALS AND METHODS: Major phenolic content from flowering aerial parts of L. spinosa was isolated and identified. The protective effects of isolated compounds (10 and 20 muM) against oxidative stress induced by tert-butyl hydroperoxide (t-BHP) in HepG2 cells were investigated through the measurement of aspartate aminotransferase (AST), alanine transaminase (ALT), and superoxide dismutase (SOD) levels. RESULTS: A new phenolic compound identified as 2,3-diferulyl R,R-(+) methyl tartrate (6), in addition to five known metabolites, esculetin (1), esculetin-7-O-d-glucoside (Cichoriin) (2), fertaric acid (3), acacetin-7-O-d-glucoside (4), and acacetin-7-O-d-glucuronic acid (5), were isolated. Oxidant-induced damage by 200 muM t-BHP in HepG2 cells was inhibited by compounds 1, 4, and 5 (10 and 20 muM), or quercetin (10 muM; positive control). The protective effects of compounds 1, 4, and 5 were associated with decreasing in AST, ALT, and SOD levels. Compound 4 (20 muM) decreased the AST level from 128.5 +/- 13.9 to 7.9 +/-1.8 U/mL. Meanwhile, compound 1 (20 muM) decreased ALT activity from 20.3 +/- 7.0 to 7.6 +/- 2.4 U/mL, while compound 5 decreased SOD levels from 41.6 +/- 9.0 to 28.3 +/- 3.4 mU/mg. CONCLUSION: The major phenolic compounds isolated from L. spinosa displayed a significant cytoprotective effect against oxidative stress, leading to maintenance of the normal redox status of the cell.
Introducing Cichorium Pumilum as a potential therapeutical agent against drug-induced benign breast tumor in rats.[Pubmed:22812448]
Electromagn Biol Med. 2012 Dec;31(4):299-309.
Cichorium Pumilum (chicory) is could be a promising cancer treatment in which a photosensitizing drug concentrates in benign tumor cells and activated by quanta at certain wavelength. Such activated extracts could lead to cell death and tumor ablation. Previous studies have shown that Cichorium Pumilum (chicory) contains photosensitive compounds such as Cichoriin, anthocyanins, lactucin, and Lactucopicrin. In the present study, the protective effect of sun light-activated Cichorium against the dimethylbenz[a]anthracene (DMBA) induced benign breast tumors to female Sprague-Dawley rats was investigated. Chicory's extract has significantly increase P.carbonyl (PC) and malondialdehyde (MDA) and decreases the hepatic levels of total antioxidant capacity (TAC) and superoxide dismutase (SOD) in benign breast tumors-induced group compared to control. It also significantly decrease the number of estrogen receptors ER-positive cells in tumor masses. These results suggest that chicory extracts could be used as herbal photosensitizing agent in treating benign breast tumor in rats.
Furofuran lignans from a callus culture of Cichorium intybus.[Pubmed:15809887]
Plant Cell Rep. 2005 Jun;24(4):246-9.
Three new and one known furofuran lignans--syringaresinol derivatives--along with the known phenylpropanoids Cichoriin and syringin were isolated from a callus tissue of Cichorium intybus. The compounds were characterised by spectral methods. This is the first report on the presence of furofuran lignans in Cichorium species.
A new coumarin glucoside ester from Cichorium intybus.[Pubmed:12385886]
Fitoterapia. 2002 Oct;73(6):544-6.
Cichoriin-6'-p-hydroxyphenyl acetate, a new natural product, was isolated from chicory leaves.
Glycosidic fraction of flue-cured tobacco leaves: its separation and component analysis.[Pubmed:10803957]
Biosci Biotechnol Biochem. 2000 Mar;64(3):584-7.
The fraction containing glycosidic components was separated from flue-cured tobacco (Nicotiana tabacum L.) leaves by a facile method. Some components of the fraction were isolated and elucidated to be syringin, coniferin, Cichoriin, benzyl-beta-D-glucoside, Blumenol A-beta-D-glucoside, and 5,6-epoxy-5,6-dihydro-3-hydroxy-beta-ionyl-beta-D-glucoside. Syringin and coniferin were detected in the Nicotiana species for the first time.
Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of Taraxacum officinale.[Pubmed:8728061]
Phytochemistry. 1996 May;42(1):121-7.
Three flavonoid glycosides: luteolin 7-glucoside and two luteolin 7-diglucosides were isolated from dandelion flowers and leaves together with free luteolin and chrysoeriol in the flower tissue. The hydroxycinnamic acids, chicoric acid, monocaffeyltartaric acid and chlorogenic acid were found throughout the plant and the coumarins, Cichoriin and aesculin were identified in the leaf extracts. This represents the first report of free chrysoeriol (luteolin 3'-methyl ether) in Taraxacum officinale agg. An earlier provisional identification of chicoric acid, chlorogenic acid, Cichoriin and aesculin in a phenolic survey of the tribe Cichorieae is confirmed. Chicoric acid and the related monocaffeyltartaric acid were found to be the major phenolic constituents in flowers, roots, leaves and involucral bracts and also in the medicinal preparations tested.


