ChalepensinCAS# 13164-03-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13164-03-9 | SDF | Download SDF |
| PubChem ID | 128834 | Appearance | Powder |
| Formula | C16H14O3 | M.Wt | 254.28 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
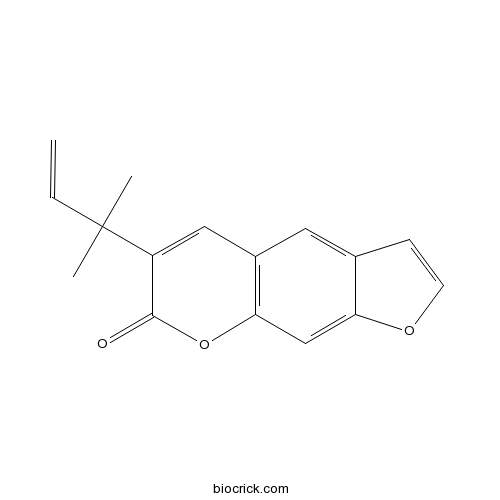
| Chemical Name | 6-(2-methylbut-3-en-2-yl)furo[3,2-g]chromen-7-one | ||
| SMILES | CC(C)(C=C)C1=CC2=C(C=C3C(=C2)C=CO3)OC1=O | ||
| Standard InChIKey | FYCCCUNGXGKNJV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14O3/c1-4-16(2,3)12-8-11-7-10-5-6-18-13(10)9-14(11)19-15(12)17/h4-9H,1H2,2-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Chalepensin shows antiprotozoal activity. 2. Chalepensin inhibits multiple P450s and that epoxidation activity is crucial for the potential drug interaction through mechanism-based inhibition. 3. Chalepensin can cause significant inhibition of radicle growth of A. hypochondriacus and E. crus-galli. 4. Chalepensin behaves as an energy transfer inhibitor at low concentration. |
| Targets | P450 (e.g. CYP17) | NADPH-oxidase | ATPase | Antifection |

Chalepensin Dilution Calculator

Chalepensin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9327 mL | 19.6634 mL | 39.3267 mL | 78.6535 mL | 98.3168 mL |
| 5 mM | 0.7865 mL | 3.9327 mL | 7.8653 mL | 15.7307 mL | 19.6634 mL |
| 10 mM | 0.3933 mL | 1.9663 mL | 3.9327 mL | 7.8653 mL | 9.8317 mL |
| 50 mM | 0.0787 mL | 0.3933 mL | 0.7865 mL | 1.5731 mL | 1.9663 mL |
| 100 mM | 0.0393 mL | 0.1966 mL | 0.3933 mL | 0.7865 mL | 0.9832 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- OPC 21268
Catalog No.:BCC7812
CAS No.:131631-89-5
- ACY-241
Catalog No.:BCC6460
CAS No.:1316215-12-9
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- (+)-Pteryxin
Catalog No.:BCN3470
CAS No.:13161-75-6
- Amyloid Beta-peptide (25-35) (human)
Catalog No.:BCC1027
CAS No.:131602-53-4
- Betulinaldehyde
Catalog No.:BCN1249
CAS No.:13159-28-9
- Calystegine A3
Catalog No.:BCN1884
CAS No.:131580-36-4
- Camelliaside B
Catalog No.:BCN3872
CAS No.:131573-90-5
- Triumbelletin
Catalog No.:BCN6779
CAS No.:131559-54-1
- VU 591 hydrochloride
Catalog No.:BCC6126
CAS No.:1315380-70-1
- NPEC-caged-(1S,3R)-ACPD
Catalog No.:BCC7653
CAS No.:1315379-60-2
- Rac1 Inhibitor F56, control peptide
Catalog No.:BCC5887
CAS No.:1315378-77-8
- Turmeronol A
Catalog No.:BCN6777
CAS No.:131651-37-1
- 8-(1-Chloro-2-hydroxy-3-methylbut-3-enyl)-7-methoxycoumarin
Catalog No.:BCN7058
CAS No.:131652-35-2
- Parsonsianine
Catalog No.:BCN2110
CAS No.:131683-36-8
- (R)-(-)-4-Benzyl-3-propionyl-2-oxazolidinone
Catalog No.:BCC8392
CAS No.:131685-53-5
- Arbidol HCl
Catalog No.:BCC3722
CAS No.:131707-23-8
- Urolignoside
Catalog No.:BCN6758
CAS No.:131723-83-6
- Flavopiridol hydrochloride
Catalog No.:BCC3925
CAS No.:131740-09-5
- DAU 5884 hydrochloride
Catalog No.:BCC7263
CAS No.:131780-48-8
- Ugaxanthone
Catalog No.:BCN6775
CAS No.:13179-11-8
- Cardenolide B-1
Catalog No.:BCN4714
CAS No.:1318158-89-2
- Aphagranin A
Catalog No.:BCN6889
CAS No.:1318173-53-3
- (R,R)-2,6-Bis(4-isopropyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8396
CAS No.:131864-67-0
Antiprotozoal activity against Entamoeba histolytica of plants used in northeast Mexican traditional medicine. Bioactive compounds from Lippia graveolens and Ruta chalepensis.[Pubmed:25517343]
Molecules. 2014 Dec 15;19(12):21044-65.
Amoebiasis caused by Entamoeba histolytica is associated with high morbidity and mortality is becoming a major public health problem worldwide, especially in developing countries. Because of the side-effects and the resistance that pathogenic protozoa build against the standard antiparasitic drugs, e.g., metronidazole, much recent attention has been paid to plants used in traditional medicine around the world in order to find new antiprotozoal agents. We collected 32 plants used in Northeast Mexican traditional medicine and the methanolic extracts of these species were screened for antiprotozoal activity against E. histolytica trophozoites using in vitro tests. Only 18 extracts showed a significant inhibiting activity and among them six plant extracts showed more than 80% growth inhibition against E. histolytica at a concentration of 150 microg/mL and the IC50 values of these extracts were determined. Lippia graveolens Kunth and Ruta chalepensis Pers. showed the more significant antiprotozoal activity (91.54% and 90.50% growth inhibition at a concentration of 150 microg/mL with IC50 values of 59.14 and 60.07 microg/mL, respectively). Bioassay-guided fractionation of the methanolic extracts from these two plants afforded carvacrol (1) and Chalepensin (2), respectively, as bioactive compounds with antiprotozoal activity.
Mechanism-based inhibition of CYP1A1 and CYP3A4 by the furanocoumarin chalepensin.[Pubmed:23257392]
Drug Metab Pharmacokinet. 2013;28(3):229-38. Epub 2012 Dec 18.
The cytochrome P450 (P450, CYP) 2A6 inhibitor Chalepensin was found to inhibit human CYP1A1, CYP1A2, CYP2A13, CYP2C9, CYP2D6, CYP2E1, and CYP3A4 to different extents. CYP1A1 and CYP3A4 underwent pronounced mechanism-based inactivation by Chalepensin and had the smallest IC50 ratios of inhibition with NADPH-fortified pre-incubation (IC50(+)) to that without pre-incubation (IC50(-)). CYP2E1 had the least susceptibility to mechanism-based inactivation. This inactivation of CYP1A1 and CYP3A4 exhibited time-dependence, led to a loss of spectrophotometrically detected P450, and could not be fully recovered by dialysis. Pre-incubation with Chalepensin did not affect NADPH-P450 reductase activity. Cytosol-supported glutathione conjugation protected CYP3A4 but not CYP1A1 against the inactivation by Chalepensin. Cytosolic decomposition of Chalepensin may contribute partially to the protection. The high epoxidation activities of CYP1A1, CYP2A6, and CYP3A4 were in agreement with their pronounced susceptibilities to mechanism-based inactivation by Chalepensin. Considering both the IC50 values and inactivation kinetic parameters, the threshold concentrations of Chalepensin for potential drug interactions through inhibition of CYP2A6 and CYP3A4 were estimated to be consistently low. These results demonstrate that Chalepensin inhibits multiple P450s and that epoxidation activity is crucial for the potential drug interaction through mechanism-based inhibition.
Allelochemicals from Stauranthus perforatus, a Rutaceous tree of the Yucatan Peninsula, Mexico.[Pubmed:15694456]
Phytochemistry. 2005 Feb;66(4):487-94.
Aqueous leachates and a CHCl3-MeOH (1:1) extract of roots of Stauranthus perforatus showed a significant phytotoxic effect on Amaranthus hypochondriacus and Echinochloa crus-galli. Bioassay-directed fractionation of the active organic extract led to the isolation and characterization of ten secondary metabolites, which included two pyranocoumarins [xanthyletin (1) and 3-(1',1'-dimethylallyl)-xanthyletin (2)], four furanocoumarins [Chalepensin (3), ammirin (4), chalepin (5) and 2'-isopropyl-psoralene (6)], two lignans [asarinin (7) and fargesin (8)], one sesquiterpene [4,5-epoxi-beta-caryophyllene (9)], and one alkamide [pellitorine (10)]. From these compounds, 2'-isopropyl-psoralene (6) or anhydromarmesin, is reported for the first time as a natural product, whereas compounds 4-10 are now reported as being present in S. perforatus. Metabolites 1, 3-5 and 10 caused significant inhibition of radicle growth of A. hypochondriacus and E. crus-galli. Furthermore, in a greenhouse experiment the decomposition of the leaves and roots in the soil had a significant inhibitory effect on the growth of weeds. The allelopathic action of the decomposition of roots was evident up to the sixth week of the experiment. The effect of leaves was comparable to that of DPCA (dimethyl tetrachloroterephthalate), a commercial herbicide. Finally different concentrations of Stauranthus root powder were combined with maize kernels and used to feed corn weevil. The treatments resulted in high mortality of this insect.
Effect of selected coumarins on spinach chloroplast photosynthesis.[Pubmed:10552509]
J Agric Food Chem. 1999 May;47(5):2137-40.
Xanthyletin (1), 3-(1',1'-dimethylallyl)xanthyletin (2), and Chalepensin (3), the major coumarins isolated from Stauranthus perforatus, inhibit ATP synthesis from water to methylviologen in spinach thylakoids in a concentration-dependent manner. At low concentration Chalepensin (3) inhibits basal and phosphorylating electron flow from water to K(3)[Fe(CN)(6)] without affecting uncoupled electron flow but accelerating Mg(2+)-ATPase activity. Thus, at low concentration the compound behaves as an energy transfer inhibitor. However, at higher concentrations this coumarin acts as an uncoupler because it enhances basal and phosphorylating electron transfer. On the other hand, coumarins 1 and 2 act as Hill reaction inhibitors, although 2 exhibited also uncoupler properties because it induces stimulation of basal and phosphorylating electron flow from water to ferricyanide. The site of interference of xanthyletin was located at the b(6)f-PC level of the electron transport chain.


