CedreloneCAS# 1254-85-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1254-85-9 | SDF | Download SDF |
| PubChem ID | 3083689 | Appearance | Powder |
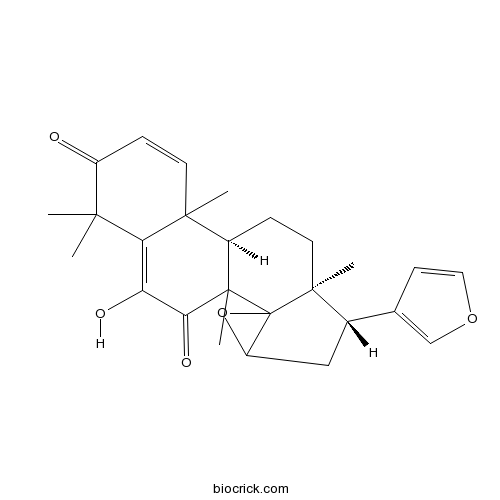
| Formula | C26H30O5 | M.Wt | 422.5 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1(C(=O)C=CC2(C1=C(C(=O)C3(C2CCC4(C35C(O5)CC4C6=COC=C6)C)C)O)C)C | ||
| Standard InChIKey | OQMUOVSEPOBWMK-MFNRWJBWSA-N | ||
| Standard InChI | InChI=1S/C26H30O5/c1-22(2)17(27)7-9-23(3)16-6-10-24(4)15(14-8-11-30-13-14)12-18-26(24,31-18)25(16,5)21(29)19(28)20(22)23/h7-9,11,13,15-16,18,28H,6,10,12H2,1-5H3/t15-,16+,18?,23?,24-,25?,26?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cedrelone exhibits antifeedant activity. 2. Cedrelone and its derivatives exert antimicrobial activities, bromohydroxy cedrelone and Michael adduct show good antifungal activity. 3. Cedrelone and dysobinin show significant cytotoxicity against cancer cell lines, such as -60, SMMC-7721, A-549, MCF-7 and SW480. 4. Cedrelone is a very potent inducers of apoptosis, it can cause cell cycle arrest. 5. Cedrelone has insecticidal activity, it inhibits arval growth of P. saucia, and the molting of the milkweed bug, Oncopeltus fasciatus. |
| Targets | MMP(e.g.TIMP) | Antifection |

Cedrelone Dilution Calculator

Cedrelone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3669 mL | 11.8343 mL | 23.6686 mL | 47.3373 mL | 59.1716 mL |
| 5 mM | 0.4734 mL | 2.3669 mL | 4.7337 mL | 9.4675 mL | 11.8343 mL |
| 10 mM | 0.2367 mL | 1.1834 mL | 2.3669 mL | 4.7337 mL | 5.9172 mL |
| 50 mM | 0.0473 mL | 0.2367 mL | 0.4734 mL | 0.9467 mL | 1.1834 mL |
| 100 mM | 0.0237 mL | 0.1183 mL | 0.2367 mL | 0.4734 mL | 0.5917 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Acetonyl-N-methyl-dihydrodecarine
Catalog No.:BCN6134
CAS No.:1253740-09-8
- AbK
Catalog No.:BCC8011
CAS No.:1253643-88-7
- NMS-E973
Catalog No.:BCC5335
CAS No.:1253584-84-7
- Antiquorin
Catalog No.:BCN7163
CAS No.:125356-08-3
- N-Debenzoyl-N-(tert-butoxycarbonyl)taxol
Catalog No.:BCN1592
CAS No.:125354-16-7
- KS 176
Catalog No.:BCC7874
CAS No.:1253452-78-6
- Periplogenin 3-[O-beta-glucopyranosyl-(1->4)-beta-sarmentopyranoside]
Catalog No.:BCN7861
CAS No.:1253421-94-1
- Pachysamine M
Catalog No.:BCN7309
CAS No.:1253202-75-3
- Vinorelbine Tartrate
Catalog No.:BCN2288
CAS No.:125317-39-7
- CD 437
Catalog No.:BCC7110
CAS No.:125316-60-1
- Ro 31-8220
Catalog No.:BCC4295
CAS No.:125314-64-9
- Chlorantholide D
Catalog No.:BCN4825
CAS No.:1253106-58-9
- TCN 238
Catalog No.:BCC7901
CAS No.:125404-04-8
- Acetate gossypol
Catalog No.:BCN5354
CAS No.:12542-36-8
- RQ-00203078
Catalog No.:BCC6419
CAS No.:1254205-52-1
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- Saclofen
Catalog No.:BCC6580
CAS No.:125464-42-8
- Sibutramine hydrochloride monohydrate
Catalog No.:BCC5251
CAS No.:125494-59-9
- SR 8278
Catalog No.:BCC6191
CAS No.:1254944-66-5
- Testosterone phenylpropionate
Catalog No.:BCC9171
CAS No.:1255-49-8
- Dracorhodin perchlorate
Catalog No.:BCN2628
CAS No.:125536-25-6
- 3',5,5',7-Tetrahydroxy-4',6-dimethoxyflavone
Catalog No.:BCN6136
CAS No.:125537-92-0
- LY 233053
Catalog No.:BCC5771
CAS No.:125546-04-5
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
Effects of limonoid cedrelone on MDA-MB-231 breast tumor cells in vitro.[Pubmed:23869780]
Anticancer Agents Med Chem. 2013 Dec;13(10):1645-53.
Cancer is the second leading cause of death, preceded only by cardiovascular diseases, and there is epidemiological evidence that demonstrate this tendency is emerging worldwide. Brazil has an extensive vegetal biodiversity with more than 55,000 species listed. Such biodiversity collaborates with the finding of compounds which could be the basis for the design of new anti-tumor drugs, with fewer side effects than the conventional chemotherapy used currently. Cedrelone is a limonoid isolated from Trichilia catigua (Meliaceae) which is a native Brazilian plant. This study demonstrates that Cedrelone inhibits proliferation, adhesion, migration and invasion of breast tumor cells from the line MDA-MB-231. The effects of cell migration and invasion on MDA-MB-231 cell may be explained, at least in part, by the ability of Cedrelone to inhibit MMP activity. We also demonstrate that Cedrelone is able to induce apoptosis in MDA-MB-231 cells. There are only a few works investigating the effect of limonoids in cellular processes closely related to tumor progression such as adhesion, migration and invasion. To the best of our knowledge, this is the first work describing the effects of a limonoid on tumor and non-tumor cell adhesion process.
Evaluation of effect of triterpenes and limonoids on cell growth, cell cycle and apoptosis in human tumor cell line.[Pubmed:21269253]
Anticancer Agents Med Chem. 2010 Dec;10(10):769-76.
Six triterpenes and eight limonoids were evaluated for their capacity to inhibit the growth of three human tumour cell lines, breast adenocarcinoma (MCF-7), non-small cell lung cancer (NCI-H460) and melanoma (A375-C5). The mechanisms involved in the observed cell growth arrest of the four most potent compounds were carried out by studying their effect in cell cycle profile and programmed cell death. The results showed that one triterpene (odoratol) and two limonoids (gedunin and Cedrelone) caused cell cycle arrest while only the limonoids gedunin and Cedrelone were found to be very potent inducers of apoptosis.
Limonoids from the leaves of Toona ciliata var. yunnanensis.[Pubmed:22277739]
Phytochemistry. 2012 Apr;76:141-9.
Twelve limonoids, toonayunnanins A-L (1-12) and eleven known compounds (13-23) were isolated from the leaves of Toona ciliata var. yunnanensis, and their structures were elucidated by means of extensive spectroscopic analyses, particularly 1D and 2D NMR techniques. The inhibitory effects of all the isolated compounds were evaluated on human tumor cell lines, such as HL-60, SMMC-7721, A-549, MCF-7 and SW480. Cedrelone (13) and dysobinin (18) showed significant cytotoxicity, and toonayunnanin B (2) and epoxyazadiradione (14), were found to be slightly cytotoxic against the above cell lines. Furthermore, this study provides valuable information for the chemotaxonomy of T. ciliata varieties.
Photooxidation of cedrelone, a tetranortriterpenoid from Toona ciliata.[Pubmed:11045715]
Photochem Photobiol. 2000 Oct;72(4):464-6.
Cedrelone, a tetranortriterpenoid on photolysis by UV light yields a true photooxidation product 3 [14 beta,15beta,22beta,23beta-diepoxy-6-hydroxy-1,5, 20(22)-meliatriene-2,7,21-trione] whose structure is well established by NMR studies and confirmed by X-ray crystallography, along with product 4 [14 beta,15beta-epoxy-6,23-dihydroxy-1,5,20(22)-meliatriene-2,7, 21-trione]. Addition of rose bengal increases the rate of photooxidation whereas DABCO decreases rate of photolysis proving the involvement of singlet oxygen in the photooxygenation. Both the photoproducts exhibited antifeedant activity.


