Cannflavin ACAS# 76735-57-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 76735-57-4 | SDF | Download SDF |
| PubChem ID | 10071695 | Appearance | Yellow powder |
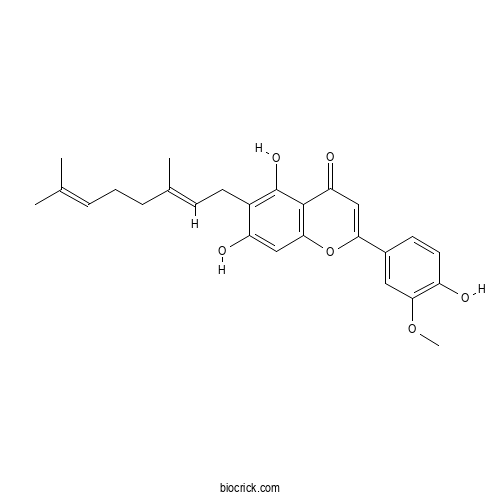
| Formula | C26H28O6 | M.Wt | 436.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Cannaflavin A; Canniflavone 2 | ||
| Solubility | Soluble in acetone | ||
| Chemical Name | 6-[(2E)-3,7-dimethylocta-2,6-dienyl]-5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)chromen-4-one | ||
| SMILES | CC(=CCCC(=CCC1=C(C2=C(C=C1O)OC(=CC2=O)C3=CC(=C(C=C3)O)OC)O)C)C | ||
| Standard InChIKey | MWGFICMOCSIQMV-LZYBPNLTSA-N | ||
| Standard InChI | InChI=1S/C26H28O6/c1-15(2)6-5-7-16(3)8-10-18-20(28)13-24-25(26(18)30)21(29)14-22(32-24)17-9-11-19(27)23(12-17)31-4/h6,8-9,11-14,27-28,30H,5,7,10H2,1-4H3/b16-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cannflavin A Dilution Calculator

Cannflavin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.291 mL | 11.4548 mL | 22.9095 mL | 45.819 mL | 57.2738 mL |
| 5 mM | 0.4582 mL | 2.291 mL | 4.5819 mL | 9.1638 mL | 11.4548 mL |
| 10 mM | 0.2291 mL | 1.1455 mL | 2.291 mL | 4.5819 mL | 5.7274 mL |
| 50 mM | 0.0458 mL | 0.2291 mL | 0.4582 mL | 0.9164 mL | 1.1455 mL |
| 100 mM | 0.0229 mL | 0.1145 mL | 0.2291 mL | 0.4582 mL | 0.5727 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Californidine perchlorate
Catalog No.:BCN0305
CAS No.:17939-31-0
- Berberine chloride dihydrate
Catalog No.:BCN0304
CAS No.:5956-60-5
- Ballonigrine
Catalog No.:BCN0303
CAS No.:62340-62-9
- Bakuchicin
Catalog No.:BCN0302
CAS No.:4412-93-5
- Avenanthramide D
Catalog No.:BCN0301
CAS No.:115610-36-1
- Atropine N-oxide hydrochloride
Catalog No.:BCN0300
CAS No.:4574-60-1
- Apoatropine hydrochloride
Catalog No.:BCN0299
CAS No.:5978-81-4
- (-)-Antofine
Catalog No.:BCN0298
CAS No.:32671-82-2
- Anisodine hydrobromide
Catalog No.:BCN0297
CAS No.:76822-34-9
- Anemonin
Catalog No.:BCN0296
CAS No.:508-44-1
- Andrograpanin
Catalog No.:BCN0295
CAS No.:82209-74-3
- L-(±)-Alliin
Catalog No.:BCN0294
CAS No.:17795-26-5
- Castalagin
Catalog No.:BCN0307
CAS No.:24312-00-3
- (-)-Catechin
Catalog No.:BCN0308
CAS No.:18829-70-4
- Convolidine
Catalog No.:BCN0309
CAS No.:63911-32-0
- trans-p-Coumaric acid
Catalog No.:BCN0310
CAS No.:501-98-4
- trans-Coutaric acid
Catalog No.:BCN0311
CAS No.:27174-07-8
- Cycloartenol
Catalog No.:BCN0312
CAS No.:469-38-5
- 5,6-Dehydro 7,8-dihydrokavain
Catalog No.:BCN0313
CAS No.:3155-51-9
- 23-epi-26-Deoxyactein
Catalog No.:BCN0314
CAS No.:501938-01-8
- Dihydroavenanthramide D
Catalog No.:BCN0315
CAS No.:697235-49-7
- 2',6'-Dihydroxy 4',4-dimethoxychalcone
Catalog No.:BCN0316
CAS No.:94441-99-3
- Diosmetin 7-glucuronide
Catalog No.:BCN0317
CAS No.:35110-20-4
- Echinatine N-oxide
Catalog No.:BCN0318
CAS No.:20267-93-0
Cannflavins - From plant to patient: A scoping review.[Pubmed:32858172]
Fitoterapia. 2020 Oct;146:104712.
INTRODUCTION: Cannflavins are a group of prenylflavonoids derived from Cannabis sativa L.. Cannflavin A (CFL-A), B (CFL-B) and C (CFL-C) have been heralded for their anti-inflammatory properties in pre-clinical evaluations. This scoping review aims to synthesise the evidence base on cannflavins to provide an overview of the current research landscape to inform research strategies to aid clinical translation. METHODS: A scoping review was conducted of EMBASE, MEDLINE, Pubmed, CENTRAL and Google Scholar databases up to 26th February 2020. All studies describing original research on cannflavins and their isomers were included for review. RESULTS: 26 full text articles were included. CFL-A and CFL-B demonstrated potent anti-inflammatory activity via inhibition of 12-o-tetradecanoylphorbol 13-acetate induced PGE2 release (CFL-A half maximal inhibitory concentration (IC50): 0.7 muM; CFL-B IC50: 0.7 muM) and microsomal prostaglandin E synthase-1 (CFL-A IC50: 1.8 muM; CFL-B IC50: 3.7 muM). Outcomes were also described in preclinical models of anti-oxidation (CFL-A), anti-parasitic activity (CFL-A, CFL-C), neuroprotection (CFL-A) and cancer (Isocannflavin B, a CFL-B isomer). In-silico screening identified that CFL-A has binding affinity with viral proteins that warrant further investigation. CONCLUSIONS: Cannflavins demonstrate a number of promising therapeutic properties, most notably as an anti-inflammatory agent. Low yields of extraction however have previously limited research to small pre-clinical investigations. Identification of cannflavin-rich chemovars, novel extraction techniques and recent identification of a biosynthetic pathway will hopefully allow research to be scaled appropriately. In order to fully evaluate the therapeutic properties of cannflavins focused research now needs to be embedded within institutions with a track-record of clinical translation.
Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS.[Pubmed:32024009]
Molecules. 2020 Jan 31;25(3). pii: molecules25030631.
Industrial hemp (Cannabis sativa L. Family Cannabaceae) contains a vast number of bioactive relevant compounds, namely polyphenols including flavonoids, phenolic acids, phenol amides, and lignanamides, well known for their therapeutic properties. Nowadays, many polyphenols-containing products made of herbal extracts are marketed, claiming to exert health-promoting effects. In this context, industrial hemp inflorescence may represent an innovative source of bioactive compounds to be used in nutraceutical formulations. The aim of this work was to provide a comprehensive analysis of the polyphenolic fraction contained in polar extracts of four different commercial cultivars (Kompoti, Tiborszallasi, Antal, and Carmagnola Cs) of hemp inflorescences through spectrophotometric (TPC, DPPH tests) and spectrometry measurement (UHPLC-Q-Orbitrap HRMS). Results highlighted a high content of Cannflavin A and B in inflorescence analyzed samples, which appear to be cannabis-specific, with a mean value of 61.8 and 84.5 mg/kg, meaning a ten-to-hundred times increase compared to other parts of the plant. Among flavonols, quercetin-3-glucoside reached up to 285.9 mg/kg in the Carmagnola CS cultivar. Catechin and epicatechin were the most representative flavanols, with a mean concentration of 53.3 and 66.2 mg/kg, respectively, for all cultivars. Total polyphenolic content in inflorescence samples was quantified in the range of 10.51 to 52.58 mg GAE/g and free radical-scavenging included in the range from 27.5 to 77.6 mmol trolox/kg. Therefore, C. sativa inflorescence could be considered as a potential novel source of polyphenols intended for nutraceutical formulations.
Novel cannabis flavonoid, cannflavin A displays both a hormetic and neuroprotective profile against amyloid beta-mediated neurotoxicity in PC12 cells: Comparison with geranylated flavonoids, mimulone and diplacone.[Pubmed:31437460]
Biochem Pharmacol. 2019 Nov;169:113609.
BACKGROUND: Flavonoids form a diverse class of naturally occurring polyphenols ascribed various biological activities, including inhibition of amyloid beta (Abeta) fibrillisation and neurotoxicity of relevance to Alzheimer's disease. Cannabis contains a unique subset of prenylated flavonoids, the cannflavins. While selected conventional flavonoids have demonstrated anti-amyloid and neuroprotective potential, any neuroprotective bioactivity of prenylated flavonoids has not been determined. We evaluated the in vitro neuroprotective and anti-aggregative properties of the novel geranylated cannabis-derived flavonoid, Cannflavin A against Abeta1-42 and compared it to two similarly geranylated flavonoids, mimulone and diplacone, to compare the bioactive properties of these unique flavonoids more broadly. METHODS: Neuronal viability were assessed in PC12 cells biochemically using the MTT assay in the presence of each flavonoid (1-200microM) for 48h. Sub-toxic threshold test concentrations of each flavonoid were then applied to cells, alone or with concomitant incubation with the lipid peroxidant tert-butyl hyrdroperoxide (t-bhp) or amyloid beta (Abeta1-42; 0-2microM). Fluorescent staining was used to indicate effects of Abeta1-42 on PC12 cellular morphology, while direct effects of each flavonoid on Abeta fibril formation and aggregation were assessed using the Thioflavin T (ThT) fluorometric kinetic assay and transmission electron microscopy (TEM) to visualise fibril and aggregate morphology. RESULTS: Cannflavin A demonstrated intrinsic hormetic effects on cell viability, increasing viability by 40% from 1 to 10microM but displaying neurotoxicity at higher (>10-100microM) concentrations. Neither mimulone nor diplacone exhibited such a biphasic effect, instead showing only concentration-dependent neurotoxicity, with diplacone the more potent (from >1microM). However at the lower concentrations (<10microM), Cannflavin A increased cell viability by up to 40%, while 10microM Cannflavin A inhibited the neurotoxicity elicited by Abeta1-42 (0-2microM), reducing Abeta aggregate adherence to PC-12 cells and associated neurite loss. The neuroprotective effects of Cannflavin A were associated with a direct inhibition of Abeta1-42 fibril and aggregate density, evidenced by attenuated ThT fluorescence kinetics and microscopic evidence of both altered and diminished density of Abeta aggregate and fibril morphology via electron microscopy. CONCLUSIONS: These findings highlight a concentration-dependent hormetic and neuroprotective role of Cannflavin A against Abeta-mediated neurotoxicity, associated with an inhibition of Abeta fibrillisation. The efficacy of the cannabis flavone may itself direct further lead development targeting neurodegeneration in Alzheimer's disease. However, the geranylated flavonoids generally displayed a comparatively potent neurotoxicity not observed with many conventional flavonoids in vitro.
Biosynthesis of cannflavins A and B from Cannabis sativa L.[Pubmed:31151063]
Phytochemistry. 2019 Aug;164:162-171.
In addition to the psychoactive constituents that are typically associated with Cannabis sativa L., there exist numerous other specialized metabolites in this plant that are believed to contribute to its medicinal versatility. This study focused on two such compounds, known as Cannflavin A and cannflavin B. These prenylated flavonoids specifically accumulate in C. sativa and are known to exhibit potent anti-inflammatory activity in various animal cell models. However, almost nothing is known about their biosynthesis. Using a combination of phylogenomic and biochemical approaches, an aromatic prenyltransferase from C. sativa (CsPT3) was identified that catalyzes the regiospecific addition of either geranyl diphosphate (GPP) or dimethylallyl diphosphate (DMAPP) to the methylated flavone, chrysoeriol, to produce cannflavins A and B, respectively. Further evidence is presented for an O-methyltransferase (CsOMT21) encoded within the C. sativa genome that specifically converts the widespread plant flavone known as luteolin to chrysoeriol, both of which accumulate in C. sativa. These results therefore imply the following reaction sequence for cannflavins A and B biosynthesis: luteolin chrysoeriol Cannflavin A and cannflavin B. Taken together, the identification of these two unique enzymes represent a branch point from the general flavonoid pathway in C. sativa and offer a tractable route towards metabolic engineering strategies that are designed to produce these two medicinally relevant Cannabis compounds.
New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp).[Pubmed:30322208]
Molecules. 2018 Oct 14;23(10). pii: molecules23102639.
Cannabis sativa L. is a dioecious plant belonging to the Cannabaceae family. The main phytochemicals that are found in this plant are represented by cannabinoids, flavones, and terpenes. Some biological activities of cannabinoids are known to be enhanced by the presence of terpenes and flavonoids in the extracts, due to a synergistic action. In the light of all the above, the present study was aimed at the multi-component analysis of the bioactive compounds present in fibre-type C. sativa (hemp) inflorescences of different varieties by means of innovative HPLC and GC methods. In particular, the profiling of non-psychoactive cannabinoids was carried out by means of HPLC-UV/DAD, ESI-MS, and MS(2). The content of prenylated flavones in hemp extracts, including cannflavins A and B, was also evaluated by HPLC. The study on Cannabis volatile compounds was performed by developing a new method based on headspace solid-phase microextraction (HS-SPME) coupled with GC-MS and GC-FID. Cannabidiolic acid (CBDA) and cannabidiol (CBD) were found to be the most abundant cannabinoids in the hemp samples analysed, while beta-myrcene and beta-caryophyllene were the major terpenes. As regards flavonoids, Cannflavin A was observed to be the main compound in almost all the samples. The methods developed in this work are suitable for the comprehensive chemical analysis of both hemp plant material and related pharmaceutical or nutraceutical products in order to ensure their quality, efficacy, and safety.
Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads.[Pubmed:28826544]
Adv Pharmacol. 2017;80:67-134.
The golden age of cannabis pharmacology began in the 1960s as Raphael Mechoulam and his colleagues in Israel isolated and synthesized cannabidiol, tetrahydrocannabinol, and other phytocannabinoids. Initially, THC garnered most research interest with sporadic attention to cannabidiol, which has only rekindled in the last 15 years through a demonstration of its remarkably versatile pharmacology and synergy with THC. Gradually a cognizance of the potential of other phytocannabinoids has developed. Contemporaneous assessment of cannabis pharmacology must be even far more inclusive. Medical and recreational consumers alike have long believed in unique attributes of certain cannabis chemovars despite their similarity in cannabinoid profiles. This has focused additional research on the pharmacological contributions of mono- and sesquiterpenoids to the effects of cannabis flower preparations. Investigation reveals these aromatic compounds to contribute modulatory and therapeutic roles in the cannabis entourage far beyond expectations considering their modest concentrations in the plant. Synergistic relationships of the terpenoids to cannabinoids will be highlighted and include many complementary roles to boost therapeutic efficacy in treatment of pain, psychiatric disorders, cancer, and numerous other areas. Additional parts of the cannabis plant provide a wide and distinct variety of other compounds of pharmacological interest, including the triterpenoid friedelin from the roots, canniprene from the fan leaves, cannabisin from seed coats, and Cannflavin A from seed sprouts. This chapter will explore the unique attributes of these agents and demonstrate how cannabis may yet fulfil its potential as Mechoulam's professed "pharmacological treasure trove."
Quality Control of Traditional Cannabis Tinctures: Pattern, Markers, and Stability.[Pubmed:28117322]
Sci Pharm. 2016 Apr 18;84(3):567-584.
Traditional tinctures of Cannabis sativa L. became obsolete before elucidation of the main cannabinoids and routine quality testing for medicines. In view of increasing medicinal use of cannabinoids and associated safety concerns, tinctures from a Delta9-tetrahydrocannabinol (THC)-type chemovar were studied. High-performance liquid chromatography with diode-array detection (HPLC/DAD) was used to determine THC, Delta9-tetrahydrocannabinolic acid A (THCA), cannabinol (CBN), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabigerol (CBG), cannabigerolic acid (CBGA), Cannflavin A/B, and total phenolics. Derived group and ratio markers describe absolute and relative profiles when varying plant part (flos, folium), extraction solvent (EtOH percentage), storage conditions ('shelf' or 'fridge' up to 15 months), and pasteurization (2 h 70 degrees C, 20 min 80 degrees C). Tinctures from female flowering tops contained ten-fold more cannabinoids than tinctures from leaves; tinctures (80%-90% EtOH) contained ten-fold more cannabinoids than tinctures (40% EtOH). The analysis of CBGA + CBG, the main co-cannabinoids aside from THCA + THC, appears more relevant than CBDA + CBD. The decarboxylation of THCA to THC-the main change during storage of freshly prepared tinctures-is after 15 months in the 'fridge' comparable to 3 months on the 'shelf'. Minimally increased CBN totals did not correlate to diminished totals of THCA and THC (up to 15% after 3 months 'shelf', 45% after 15 months 'fridge'). Instead, total cannabinoids or acidic/neutral cannabinoid ratios are better stability markers. Moderate changes after pasteurization and partial losses below 10% for total cannabinoids after 9 months 'fridge' indicate possibilities for a reasonable shelf life. Yet storage and use of non-stabilized tinctures remain critical without authorized specification and stability data because a consistent cannabinoid content is not guaranteed.
(1)H NMR and HPLC/DAD for Cannabis sativa L. chemotype distinction, extract profiling and specification.[Pubmed:26048837]
Talanta. 2015 Aug 1;140:150-165.
The medicinal use of different chemovars and extracts of Cannabis sativa L. requires standardization beyond 9-tetrahydrocannabinol (THC) with complementing methods. We investigated the suitability of (1)H NMR key signals for distinction of four chemotypes measured in deuterated dimethylsulfoxide together with two new validated HPLC/DAD methods used for identification and extract profiling based on the main pattern of cannabinoids and other phenolics alongside the assayed content of THC, cannabidiol (CBD), cannabigerol (CBG) their acidic counterparts (THCA, CBDA, CBGA), cannabinol (CBN) and Cannflavin A and B. Effects on cell viability (MTT assay, HeLa) were tested. The dominant cannabinoid pairs allowed chemotype recognition via assignment of selective proton signals and via HPLC even in cannabinoid-low extracts from the THC, CBD and CBG type. Substantial concentrations of cannabinoid acids in non-heated extracts suggest their consideration for total values in chemotype distinction and specifications of herbal drugs and extracts. Cannflavin A/B are extracted and detected together with cannabinoids but always subordinated, while other phenolics can be accumulated via fractionation and detected in a wide fingerprint but may equally serve as qualitative marker only. Cell viability reduction in HeLa was more determined by the total cannabinoid content than by the specific cannabinoid profile. Therefore the analysis and labeling of total cannabinoids together with the content of THC and 2-4 lead cannabinoids are considered essential. The suitability of analytical methods and the range of compound groups summarized in group and ratio markers are discussed regarding plant classification and pharmaceutical specification.
Antiparasitic activity of C-geranyl flavonoids from Mimulus bigelovii.[Pubmed:21796699]
Phytother Res. 2011 Aug;25(8):1246-9.
Bioactivity-directed fractionation of the MeOH fraction of the extract of Mimulus bigelovii by means of an axenic Leishmania amastigote assay and chromatographic techniques resulted in the isolation of four C-geranyl flavanones, diplacone (1), 3'-O-methyldiplacone (2), 4'-O-methyldiplacone (3), 3'-O-methyldiplacol (4), together with a geranylated flavone, Cannflavin A (5). These compounds were separated from M. bigelovii for the first time. All compounds showed moderate antileishmanial activity against axenic Leishmania donovani amastigotes with IC(50) values ranging from 4.8 to 14.6 mug/mL. The compounds were also tested against the related kinetoplastid parasite Trypanosoma brucei brucei and they showed activity with IC(50) values ranging from 1.4 to 7.2 mug/mL.
Microbial metabolism of cannflavin A and B isolated from Cannabis sativa.[Pubmed:20223485]
Phytochemistry. 2010 Jun;71(8-9):1014-9.
Microbial metabolism of Cannflavin A (1) and B (2), two biologically active flavonoids isolated from Cannabis sativa L., produced five metabolites (3-7). Incubation of 1 and 2 with Mucor ramannianus (ATCC 9628) and Beauveria bassiana (ATCC 13144), respectively, yielded 6''S,7''-dihydroxyCannflavin A (3), 6''S,7''-dihydroxyCannflavin A 7-sulfate (4) and 6''S,7''-dihydroxyCannflavin A 4'-O-alpha-L-rhamnopyranoside (5), and cannflavin B 7-O-beta-D-4'''-O-methylglucopyranoside (6) and cannflavin B 7-sulfate (7), respectively. All compounds were evaluated for antimicrobial and antiprotozoal activity.
Non-cannabinoid constituents from a high potency Cannabis sativa variety.[Pubmed:18774146]
Phytochemistry. 2008 Oct;69(14):2627-33.
Six new non-cannabinoid constituents were isolated from a high potency Cannabis sativa L. variety, namely 5-acetoxy-6-geranyl-3-n-pentyl-1,4-benzoquinone (1), 4,5-dihydroxy-2,3,6-trimethoxy-9,10-dihydrophenanthrene (2), 4-hydroxy-2,3,6,7-tetramethoxy-9,10-dihydrophenanthrene (3), 4,7-dimethoxy-1,2,5-trihydroxyphenanthrene (4), cannflavin C (5) and beta-sitosteryl-3-O-beta-d-glucopyranoside-2'-O-palmitate (6). In addition, five known compounds, alpha-cannabispiranol (7), chrysoeriol (8), 6-prenylapigenin (9), Cannflavin A (10) and beta-acetyl cannabispiranol (11) were identified, with 8 and 9 being reported for the first time from cannabis. Some isolates displayed weak to strong antimicrobial, antileishmanial, antimalarial and anti-oxidant activities. Compounds 2-4 were inactive as analgesics.
NMR assignments of the major cannabinoids and cannabiflavonoids isolated from flowers of Cannabis sativa.[Pubmed:15595449]
Phytochem Anal. 2004 Nov-Dec;15(6):345-54.
The complete 1H- and 13C-NMR assignments of the major Cannabis constituents, delta9-tetrahydrocannabinol, tetrahydrocannabinolic acid, delta8-tetrahydrocannabinol, cannabigerol, cannabinol, cannabidiol, cannabidiolic acid, Cannflavin A and cannflavin B have been determined on the basis of one- and two-dimensional NMR spectra including 1H- and 13C-NMR, 1H-1H-COSY, HMQC and HMBC. The substitution of carboxylic acid on the cannabinoid nucleus (as in tetrahydrocannabinolic acid and cannabidiolic acid) has a large effect on the chemical shift of H-1" of the C5 side chain and 2'-OH. It was also observed that carboxylic acid substitution reduces intermolecular hydrogen bonding resulting in a sharpening of the H-5' signal in cannabinolic acid in deuterated chloroform. The additional aromaticity of cannabinol causes the two angular methyl groups (H-8 and H-9) to show identical 1H-NMR shifts, which indicates that the two aromatic rings are in one plane in contrast to the other cannabinoids. For the cannabiflavonoids, the unambiguous assignments of C-3' and C-4' of Cannflavin A and B were determined by HMBC spectra.


