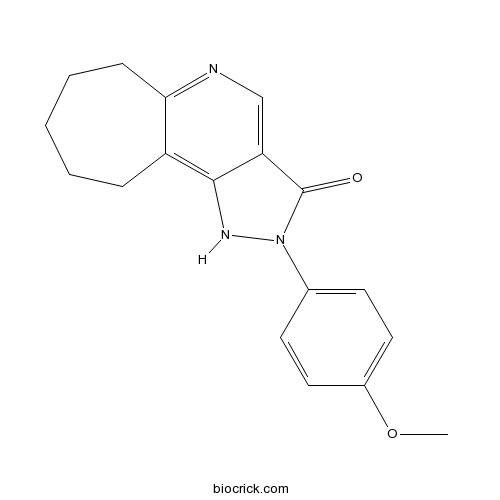
CGS 20625CAS# 111205-55-1 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111205-55-1 | SDF | Download SDF |
| PubChem ID | 163844 | Appearance | Powder |
| Formula | C18H19N3O2 | M.Wt | 309.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| SMILES | COC1=CC=C(C=C1)N2C(=O)C3=CN=C4CCCCCC4=C3N2 | ||
| Standard InChIKey | UBLXQFIFWUEVGJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H19N3O2/c1-23-13-9-7-12(8-10-13)21-18(22)15-11-19-16-6-4-2-3-5-14(16)17(15)20-21/h7-11,20H,2-6H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective, partial agonist for the benzodiazepine binding site of the GABAA receptor. Potently inhibits [3H]-flunitrazepam binding to central benzodiazepine receptors (IC50 = 1.3 nM) and displays weak affinity for peripheral benzodiazepine (IC50 = 0.68 - 2.25 μM) and GABA binding sites (IC50 > 10000 μM). Displays anxiolytic activity in vivo following oral administration.. |

CGS 20625 Dilution Calculator

CGS 20625 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2325 mL | 16.1624 mL | 32.3248 mL | 64.6496 mL | 80.812 mL |
| 5 mM | 0.6465 mL | 3.2325 mL | 6.465 mL | 12.9299 mL | 16.1624 mL |
| 10 mM | 0.3232 mL | 1.6162 mL | 3.2325 mL | 6.465 mL | 8.0812 mL |
| 50 mM | 0.0646 mL | 0.3232 mL | 0.6465 mL | 1.293 mL | 1.6162 mL |
| 100 mM | 0.0323 mL | 0.1616 mL | 0.3232 mL | 0.6465 mL | 0.8081 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7,3',4'-Trihydroxy-3-benzyl-2H-chromene
Catalog No.:BCN1621
CAS No.:1111897-60-9
- 1,2-O-Dilinoleoyl-3-O-beta-D-galactopyranosylracglycerol
Catalog No.:BCN6768
CAS No.:111187-15-6
- PF-04880594
Catalog No.:BCC3998
CAS No.:1111636-35-1
- NF 110
Catalog No.:BCC7404
CAS No.:111150-22-2
- 14-Hydroxy sprengerinin C
Catalog No.:BCN2777
CAS No.:1111088-89-1
- FERb 033
Catalog No.:BCC7701
CAS No.:1111084-78-6
- Dacomitinib (PF299804, PF299)
Catalog No.:BCC3683
CAS No.:1110813-31-4
- Fmoc-Ser(Trt)-OH
Catalog No.:BCC3546
CAS No.:111061-56-4
- Fmoc-D-Lys(Trt)-OH
Catalog No.:BCC2594
CAS No.:111061-54-2
- N-Benzoyl-2-hydroxy-2-phenylethylamine
Catalog No.:BCN1622
CAS No.:111059-46-2
- Ginkgolic acid C17:1
Catalog No.:BCN5334
CAS No.:111047-30-4
- 2-(2'-Hydroxy-4'-methylphenyl)propionic acid
Catalog No.:BCN7980
CAS No.:111044-84-9
- Episappanol
Catalog No.:BCN7940
CAS No.:111254-18-3
- Sappanol
Catalog No.:BCN3735
CAS No.:111254-19-4
- Lestaurtinib
Catalog No.:BCC2440
CAS No.:111358-88-4
- Zileuton
Catalog No.:BCC2515
CAS No.:111406-87-2
- Azadirachtin
Catalog No.:BCC8123
CAS No.:11141-17-6
- Pinocembrin diacetate
Catalog No.:BCN5997
CAS No.:111441-88-4
- Axinysone B
Catalog No.:BCN7713
CAS No.:1114491-60-9
- Naltrindole hydrochloride
Catalog No.:BCC6773
CAS No.:111469-81-9
- Amlodipine Besylate
Catalog No.:BCC4397
CAS No.:111470-99-6
- 25-Anhydroalisol F
Catalog No.:BCN3361
CAS No.:1114895-01-0
- Ac-DL-Met-OH
Catalog No.:BCC2999
CAS No.:1115-47-5
- H-Ala-OEt.HCl
Catalog No.:BCC2687
CAS No.:1115-59-9
Determination of a potential anxiolytic drug (CGS 20625) in human plasma by high-performance liquid chromatography.[Pubmed:1686029]
J Chromatogr. 1991 Aug 23;568(2):487-93.
An analytical method employing reversed-phase high-performance liquid chromatography is described for the determination of a potential anxiolytic agent in human plasma. This experimental drug candidate has potent and selective affinity for the central benzodiazepine receptor complex. The compound and internal standard are extracted from buffered plasma (pH 9.0) into ethyl acetate. The solvent is evaporated and the residue is reconstituted in chromatographic mobile phase. Separation is achieved on a 5-microns phenyl column with ultraviolet absorbance detection of the drug and internal standard at 270 nm. Recovery and reproducibility assessments indicate good accuracy (overall relative recovery of 101%) and precision (coefficients of variation from 2.0 to 11%) over the concentration range 10-1000 ng/ml. The limit of quantification for the method is 10 ng/ml. The method is suitable for pharmacokinetic analysis following the administration of 80 mg of drug to normal volunteers.
Oral absorption of CGS-20625, an insoluble drug, in dogs and man.[Pubmed:8576841]
J Pharmacokinet Biopharm. 1995 Feb;23(1):11-23.
Oral bioavailability of highly water-insoluble drugs is often quite limited and variable, requiring the development of improved formulations. Animal models are an essential aspect of the design and testing of such formulations designed to improve absorption in man. The present report compares the absorption of CGS-20625, an insoluble drug, in dog and man after oral administration of the drug as a powder, a solid dispersion capsule, and after gastric and duodenal administration in PEG 400 solution. CGS-20625 powder (20 mg) given orally exhibited slow, delayed absorption in both dog and man, with a Cmax of 0.26 +/- 0.07 microgram/ml at Tmax of 3 hr in dog, and 0.01 +/- 0.004 microgram/ml at 2 hr in man. Administration of CGS-20625 in PEG 400 solution improved absorption in dog and man, with a Cmax of 1.2 +/- 0.10 microgram/ml at Tmax of 0.25 hr in dog, and a Cmax of 0.10 +/- 0.04 microgram/ml at 0.5 hr in man. Tmax after administration of the hard gelatin capsule formulation was 0.9 and 1.0 hr in dog and man, with Cmax of 0.89 +/- 0.16 and 0.052 +/- 0.014 microgram/ml, respectively. Absolute bioavailability of CGS-20625 powder in the dog was 0.67 +/- 0.21, whereas the bioavailabilities of the powder and the capsule relative to the PEG 400 solution were 0.84 and 1.1, respectively, in dog, and 0.41 and 0.85 respectively, in man. No significant benefits of duodenal administration were observed. Plasma levels were approximately 10-fold greater and oral clearance was approximately 5-fold less in the dog than in man. Furthermore, pharmacokinetic data were less variable and relative bioavailability was greater in dogs than in humans. Physiological factors in the gastrointestinal tract or greater first-pass metabolism in man may account for these species differences. The relative rate and extent of CGS-20625 absorption were similar between dog and man, in the order of powder < capsule < PEG 400 solution. In addition, in vivo absorption rates in both species reflect in vitro dissolution differences between the powder and the capsule. These data strongly support the use of the dog as a model for developing improved formulations of CGS-20625. Further investigation of the dog as a model to evaluate insoluble drug absorption is warranted.
CGS 20625, a novel pyrazolopyridine anxiolytic.[Pubmed:2563294]
J Pharmacol Exp Ther. 1989 Jan;248(1):89-96.
CGS 20625 (2-(4-methoxyphenyl)2,3,5,6,7,8,9,10-octa hydrocyclohepta[b]pyrazolo-[3,4-d]pyridin-3-one) is a potent and selective ligand for the central benzodiazepine receptor (IC50 = 1.3 nM), with little or no affinity to several other neurotransmitter receptor binding sites in vitro. CGS 20625 had a gamma-aminobutyric acid ratio of 0.9 and increased t-[35S]butylbicyclophosphorothionate binding by 20% in vitro, a profile indicative of a partial agonist or mixed agonist/antagonist. In vivo, CGS 20625 blocked a pentylenetetrazol discriminative cue with an ED50 = 1.7 mg/kg p.o. The compound selectively increased conflict responding in the Cook-Davidson paradigm with a minimal effective dose of 0.3 mg/kg p.o., as compared with 3.0 mg/kg p.o. for diazepam. At doses as high as 100 mg/kg p.o., CGS 20625 had no effect on variable interval responding, suggesting minimal sedation. Unlike diazepam, CGS 20625 had no effect on rotorod performance at doses up to 100 mg/kg p.o. indicating no overt muscle relaxation, and did not potentiate the action of ethanol in this behavioral paradigm. Also, CGS 20625 had no marked effect on locomotor behavior, did not potentiate hexobarbital sleep time and had no sedative activity at doses up to 300 mg/kg p.o. CGS 20625 was efficacious in preventing pentylenetetrazol-induced seizures (ED50 = 0.7 mg/kg p.o.), had less efficacy with no clear dose-response relationship against picrotoxin-induced seizures and had no effect on either strychnine or electroshock-induced convulsions at doses up to 300 mg/kg p.o.(ABSTRACT TRUNCATED AT 250 WORDS)


