CCT244747Potent and selective CHK1 inhibitor CAS# 1404095-34-6 |
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- AZD7762
Catalog No.:BCC2555
CAS No.:860352-01-8
- MK-8776 (SCH-900776)
Catalog No.:BCC3817
CAS No.:891494-63-6
- LY2603618
Catalog No.:BCC3923
CAS No.:911222-45-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1404095-34-6 | SDF | Download SDF |
| PubChem ID | 54758482 | Appearance | Powder |
| Formula | C20H24N8O2 | M.Wt | 408.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (244.82 mM; Need ultrasonic) | ||
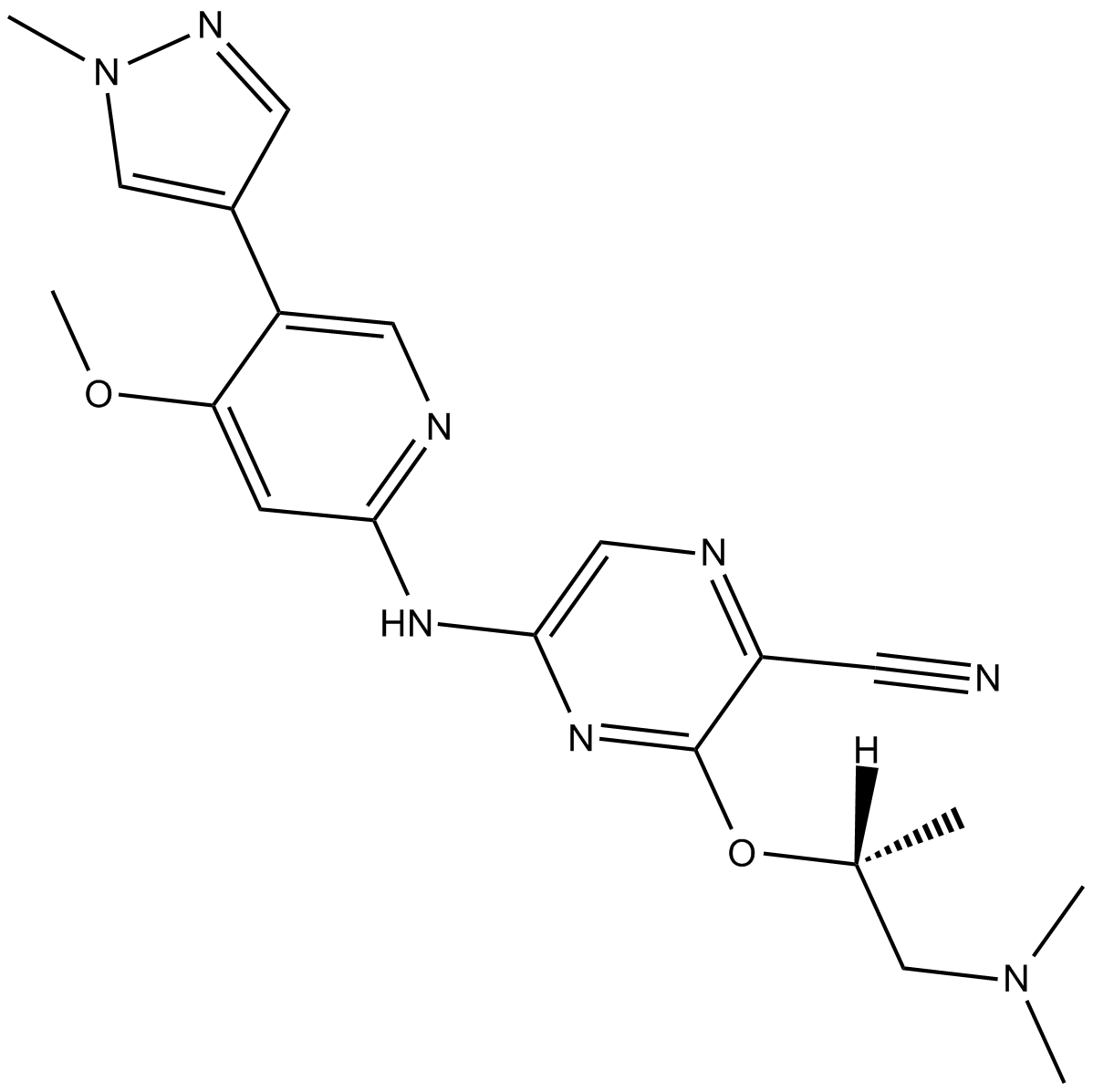
| Chemical Name | 3-[(2R)-1-(dimethylamino)propan-2-yl]oxy-5-[[4-methoxy-5-(1-methylpyrazol-4-yl)pyridin-2-yl]amino]pyrazine-2-carbonitrile | ||
| SMILES | CC(CN(C)C)OC1=NC(=CN=C1C#N)NC2=NC=C(C(=C2)OC)C3=CN(N=C3)C | ||
| Standard InChIKey | IENLGMOXAQMNEH-CYBMUJFWSA-N | ||
| Standard InChI | InChI=1S/C20H24N8O2/c1-13(11-27(2)3)30-20-16(7-21)22-10-19(26-20)25-18-6-17(29-5)15(9-23-18)14-8-24-28(4)12-14/h6,8-10,12-13H,11H2,1-5H3,(H,23,25,26)/t13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CCT244747 is a potent, orally bioavailable and highly selective CHK1 inhibitor, with an IC50 of 7.7 nM; CCT244747 also abrogates G2 checkpoint with an IC50 of 29 nM.In Vitro:CCT244747 poorly inhibits CHK2 (IC50 >10 μM) and CDK1 (IC50 >10 μM). CCT244747 has potent activities against CHK1, RSK1, RSK2, AMPK, BRSK1, IRAK1,and TrkA, with >80% inhibition. CCT244747 (10 μM) exhibits <25% inhibition of the other ion channels including hNav1.5, hKv4.3/hKChIP2, hCav1.2, hKv1.5, hKCNQ1/hminK, hHCN4[1]. CCT244747 inhibits FLT3 with an IC50 of 600 nM. CCT244747 (0.5 μM) overcomes genotoxic-induced S and G2 cell cycle arrest in human colon cancer cell lines. CCT244747 inhibits cellular CHK1 function with IC50s ranging from 29 nM to 170 nM for cellular G2 checkpoint abrogation (MIA, mitosis induction assay) in HT29, SW620, MiaPaCa-2, and Calu6 cell lines; the GI50s are between 0.33 and 3μM. CCT244747 (0.3 μM) inhibits SN38 and gemcitabine-induced CHK1 activity in HT29 and SW620 colon cancer cell lines and this correlates with abrogation of cell cycle arrest, induction of DNA damage and apoptosis[2]. CCT244747 (0.5-2.0 μM) increases the sensitivity of bladder and head and neck cancer cell lines (T24, RT112 and Cal27) to radiation[3].In Vivo:CCT244747 (100 mg/kg po, qd7d) significantly reduces tumor burden in human tumor xenografts. CCT244747 (100-300 mg/kg, po) inhibits gemcitabine-induced pS296 CHK1 for up to 24 h in HT29 colon tumor xenografts[1]. CCT244747 (75 mg/kg, p.o.) in combination with gemcitabine has potent antitumor effects in HT29 colon tumor xenografts and Calu6 human lung cancer xenografts. CCT244747 (150 mg/kg p.o.) also shows antitumor activities with irinotecan in SW620 human colon tumor xenografts[2]. CCT244747 (100 mg/kg, p.o.) exhibits radiosensitization activity in Cal27 xenografts[3]. References: | |||||

CCT244747 Dilution Calculator

CCT244747 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4482 mL | 12.2411 mL | 24.4822 mL | 48.9644 mL | 61.2055 mL |
| 5 mM | 0.4896 mL | 2.4482 mL | 4.8964 mL | 9.7929 mL | 12.2411 mL |
| 10 mM | 0.2448 mL | 1.2241 mL | 2.4482 mL | 4.8964 mL | 6.1206 mL |
| 50 mM | 0.049 mL | 0.2448 mL | 0.4896 mL | 0.9793 mL | 1.2241 mL |
| 100 mM | 0.0245 mL | 0.1224 mL | 0.2448 mL | 0.4896 mL | 0.6121 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description:
IC50: 29-170 nM
CHK1 is a serine/threonine kinase that is activated in response to single strand breaks (SSBs) in DNA caused by either direct DNA damage or replication stress. Activation of CHK1 initiates a signaling cascade culminating in cell cycle arrest leading to DNA repair, senescence or death. Inhibition of CHK1 abrogates cell cycle arrest, inhibits DNA repair and induces tumor cell death following DNA damage by a range of chemotherapeutic agents. CCT244747 is a potent and selective CHK1 inhibitor.
In vitro: CCT244747 inhibited cellular CHK1 activity, significantly enhanced the cytotoxicity of a few anticancer drugs and abrogated drug-induced S and G2 arrest in multiple tumor cell lines. Biomarkers of CHK1 activity and cell cycle inactivity were induced by genotoxics and inhibited by CCT244747, producing enhanced DNA damage and apoptosis [1].
In vivo: Active tumor concentrations of CCT244747 were obtained following oral administration. The antitumor activities of both gemcitabine and irinotecan were significantly enhanced by CCT244747 in human tumor xenografts, giving concomitant biomarker modulation indicative of CHK1 inhibition [1].
Clinical trial: Up to now, CCT244747 is still in the preclinical development stage.
Reference:
[1] Walton MI, Eve PD, Hayes A, Valenti MR, De Haven Brandon AK, Box G, Hallsworth A, Smith EL, Boxall KJ, Lainchbury M, Matthews TP, Jamin Y, Robinson SP, Aherne GW, Reader JC, Chesler L, Raynaud FI, Eccles SA, Collins I, Garrett MD. CCT244747 is a novel potent and selective CHK1 inhibitor with oral efficacy alone and in combination with genotoxic anticancer drugs. Clin Cancer Res. 2012 Oct 15;18(20):5650-61.
- RSVA 405
Catalog No.:BCC8016
CAS No.:140405-36-3
- Vancomycin hydrochloride
Catalog No.:BCC4232
CAS No.:1404-93-9
- 6'-O-Cinnamoyl-8-epikingisidic acid
Catalog No.:BCN7059
CAS No.:1403984-03-1
- Nexturastat A
Catalog No.:BCC5345
CAS No.:1403783-31-2
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
- Heteroclitin D
Catalog No.:BCN8166
CAS No.:140369-76-2
- Macranthoidin A
Catalog No.:BCN2808
CAS No.:140360-29-8
- EPZ-6438
Catalog No.:BCC3634
CAS No.:1403254-99-8
- Squalene-2,3-diol
Catalog No.:BCN6220
CAS No.:14031-37-9
- Chetomin
Catalog No.:BCC2432
CAS No.:1403-36-7
- NLG919
Catalog No.:BCC2325
CAS No.:1402836-58-1
- N-Methyllindcarpine
Catalog No.:BCN6218
CAS No.:14028-97-8
- ON 146040
Catalog No.:BCC8058
CAS No.:1404231-34-0
- 7-Methoxycoumarin-4-acetyl-P-L-G-L-β-(2,4-dinitrophenylamino)A-R amide
Catalog No.:BCC1086
CAS No.:140430-53-1
- Fmoc-Dap(Dnp)-OH
Catalog No.:BCC2666
CAS No.:140430-54-2
- ML 281
Catalog No.:BCC6317
CAS No.:1404437-62-2
- GSK 2830371
Catalog No.:BCC4179
CAS No.:1404456-53-6
- 11-Hydroxyjasmonic acid
Catalog No.:BCN6221
CAS No.:140447-14-9
- Ergosterol peroxide glucoside
Catalog No.:BCN6222
CAS No.:140447-22-9
- Heteroclitin C
Catalog No.:BCN3632
CAS No.:140460-42-0
- Heteroclitin B
Catalog No.:BCN3745
CAS No.:140461-47-8
- Olopatadine HCl
Catalog No.:BCC4545
CAS No.:140462-76-6
- Neomycin sulfate
Catalog No.:BCC4682
CAS No.:1405-10-3
- Capreomycin Sulfate
Catalog No.:BCC4644
CAS No.:1405-37-4
CCT244747 is a novel potent and selective CHK1 inhibitor with oral efficacy alone and in combination with genotoxic anticancer drugs.[Pubmed:22929806]
Clin Cancer Res. 2012 Oct 15;18(20):5650-61.
PURPOSE: Many tumors exhibit defective cell-cycle checkpoint control and increased replicative stress. CHK1 is critically involved in the DNA damage response and maintenance of replication fork stability. We have therefore discovered a novel potent, highly selective, orally active ATP-competitive CHK1 inhibitor, CCT244747, and present its preclinical pharmacology and therapeutic activity. EXPERIMENTAL DESIGN: Cellular CHK1 activity was assessed using an ELISA assay, and cytotoxicity a SRB assay. Biomarker modulation was measured using immunoblotting, and cell-cycle effects by flow cytometry analysis. Single-agent oral CCT244747 antitumor activity was evaluated in a MYCN-driven transgenic mouse model of neuroblastoma by MRI and in genotoxic combinations in human tumor xenografts by growth delay. RESULTS: CCT244747 inhibited cellular CHK1 activity (IC(50) 29-170 nmol/L), significantly enhanced the cytotoxicity of several anticancer drugs, and abrogated drug-induced S and G(2) arrest in multiple tumor cell lines. Biomarkers of CHK1 (pS296 CHK1) activity and cell-cycle inactivity (pY15 CDK1) were induced by genotoxics and inhibited by CCT244747 both in vitro and in vivo, producing enhanced DNA damage and apoptosis. Active tumor concentrations of CCT244747 were obtained following oral administration. The antitumor activity of both gemcitabine and irinotecan were significantly enhanced by CCT244747 in several human tumor xenografts, giving concomitant biomarker modulation indicative of CHK1 inhibition. CCT244747 also showed marked antitumor activity as a single agent in a MYCN-driven neuroblastoma. CONCLUSION: CCT244747 represents the first structural disclosure of a highly selective, orally active CHK1 inhibitor and warrants further evaluation alone or combined with genotoxic anticancer therapies.
An orally bioavailable Chk1 inhibitor, CCT244747, sensitizes bladder and head and neck cancer cell lines to radiation.[Pubmed:28131548]
Radiother Oncol. 2017 Mar;122(3):470-475.
PURPOSE: Chk1 inhibition increases cell sensitivity to both chemotherapy and radiotherapy in several tumour types and is, therefore, a promising anti-cancer approach. Although several Chk1 inhibitors have been developed, their clinical progress has been hampered by low bioavailability and off-target toxicities. MATERIALS AND METHODS: We characterized the radiosensitizing activity of CCT244747, the first orally bioavailable Chk1 inhibitor. We used a panel of bladder and head and neck cancer cell lines and monitored the effect of combining CCT244747 with radiation both in in vitro and in vivo models. RESULTS: CCT244747 sensitized cancer cell lines to radiation in vitro and resulted in a growth delay in cancer xenograft models associated with a survival benefit. Radiosensitization was elicited by abrogation of the radiation-induced G2 arrest and premature entry into mitosis. CONCLUSIONS: CCT244747 is a potent and specific Chk1 inhibitor that can be administered orally. It radiosensitizes tumour cell lines and represents a new therapy for clinical application in combination with radiotherapy.


