Boc-Pro-OHCAS# 15761-39-4 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

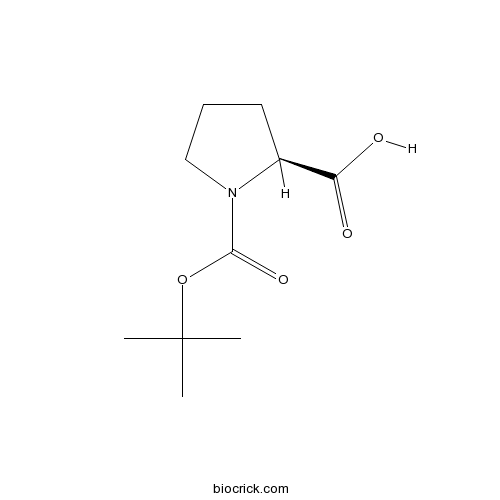
| Cas No. | 15761-39-4 | SDF | Download SDF |
| PubChem ID | 85083 | Appearance | Powder |
| Formula | C10H17NO4 | M.Wt | 215.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Boc-L-Proline;N-Boc-L-Proline; N-(Tert-Butoxycarbonyl)-L-Proline | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid | ||
| SMILES | CC(C)(C)OC(=O)N1CCCC1C(=O)O | ||
| Standard InChIKey | ZQEBQGAAWMOMAI-ZETCQYMHSA-N | ||
| Standard InChI | InChI=1S/C10H17NO4/c1-10(2,3)15-9(14)11-6-4-5-7(11)8(12)13/h7H,4-6H2,1-3H3,(H,12,13)/t7-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-Pro-OH Dilution Calculator

Boc-Pro-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6447 mL | 23.2234 mL | 46.4468 mL | 92.8936 mL | 116.117 mL |
| 5 mM | 0.9289 mL | 4.6447 mL | 9.2894 mL | 18.5787 mL | 23.2234 mL |
| 10 mM | 0.4645 mL | 2.3223 mL | 4.6447 mL | 9.2894 mL | 11.6117 mL |
| 50 mM | 0.0929 mL | 0.4645 mL | 0.9289 mL | 1.8579 mL | 2.3223 mL |
| 100 mM | 0.0464 mL | 0.2322 mL | 0.4645 mL | 0.9289 mL | 1.1612 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-Pro-OH
- Boc-Ala-OH
Catalog No.:BCC3047
CAS No.:15761-38-3
- (S)-SNAP 5114
Catalog No.:BCC7117
CAS No.:157604-55-2
- BTS
Catalog No.:BCC5425
CAS No.:1576-37-0
- Coronarin D methyl ether
Catalog No.:BCN1705
CAS No.:157528-81-9
- Taxuspine B
Catalog No.:BCN6938
CAS No.:157414-05-6
- Bacoside A3
Catalog No.:BCC8128
CAS No.:157408-08-7
- MAP4
Catalog No.:BCC6758
CAS No.:157381-42-5
- DMP 777
Catalog No.:BCC1534
CAS No.:157341-41-8
- Travoprost
Catalog No.:BCC5189
CAS No.:157283-68-6
- ML-323
Catalog No.:BCC4313
CAS No.:1572414-83-5
- Bosentan Hydrate
Catalog No.:BCC4494
CAS No.:157212-55-0
- 3-Amino-5-phenylpyrazole
Catalog No.:BCC8616
CAS No.:1572-10-7
- NSC 632839 hydrochloride
Catalog No.:BCC2088
CAS No.:157654-67-6
- Prenylpiperitol
Catalog No.:BCN1706
CAS No.:157659-20-6
- 7-Xylosyl-10-deacetylbaccatin III
Catalog No.:BCN7668
CAS No.:157664-03-4
- Perifosine
Catalog No.:BCC3673
CAS No.:157716-52-4
- Gentiournoside D
Catalog No.:BCN7855
CAS No.:157722-21-9
- Gypenoside A
Catalog No.:BCN8459
CAS No.:157752-01-7
- 5-Nonyloxytryptamine oxalate
Catalog No.:BCC6839
CAS No.:157798-13-5
- 5-Benzimidazolecarboxylic acid
Catalog No.:BCC8739
CAS No.:15788-16-6
- (Z)-2-decenoic acid
Catalog No.:BCC1295
CAS No.:15790-91-7
- Chitinase-IN-1
Catalog No.:BCC5533
CAS No.:1579991-61-9
- Chitinase-IN-2
Catalog No.:BCC5534
CAS No.:1579991-63-1
- ortho-iodoHoechst 33258
Catalog No.:BCC1824
CAS No.:158013-41-3
A convergent solution-phase synthesis of the macrocycle Ac-Phe-[Orn-Pro-D-Cha-Trp-Arg], a potent new antiinflammatory drug.[Pubmed:12762752]
J Org Chem. 2003 May 30;68(11):4464-71.
Relatively few cyclic peptides have reached the pharmaceutical marketplace during the past decade, most produced through fermentation rather than made synthetically. Generally, this class of compounds is synthesized for research purposes on milligram scales by solid-phase methods, but if the potential of macrocyclic peptidomimetics is to be realized, low-cost larger scale solution-phase syntheses need to be devised and optimized to provide sufficient quantities for preclinical, clinical, and commercial uses. Here, we describe a cheap, medium-scale, solution-phase synthesis of the first reported highly potent, selective, and orally active antagonist of the human C5a receptor. This compound, Ac-Phe[Orn-Pro-d-Cha-Trp-Arg], known as 3D53, is a macrocyclic peptidomimetic of the human plasma protein C5a and displays excellent antiinflammatory activity in numerous animal models of human disease. In a convergent approach, two tripeptide fragments Ac-Phe-Orn(Boc)-Pro-OH and H-d-Cha-Trp(For)-Arg-OEt were first prepared by high-yielding solution-phase couplings using a mixed anhydride method before coupling them to give a linear hexapeptide which, after deprotection, was obtained in 38% overall yield from the commercially available amino acids. Cyclization in solution using BOP reagent gave the antagonist in 33% yield (13% overall) after HPLC purification. Significant features of the synthesis were that the Arg side chain was left unprotected throughout, the component Boc-d-Cha-OH was obtained very efficiently via hydrogenation of d-Phe with PtO(2) in TFA/water, the tripeptides were coupled at the Pro-Cha junction to minimize racemization via the oxazolone pathway, and the entire synthesis was carried out without purification of any intermediates. The target cyclic product was purified (>97%) by reversed-phase HPLC. This convergent synthesis with minimal use of protecting groups allowed batches of 50-100 g to be prepared efficiently in high yield using standard laboratory equipment. This type of procedure should be useful for making even larger quantities of this and other macrocyclic peptidomimetic drugs.
Synthesis, radiolabeling and biological activity of peptide oostatic hormone and its analogues.[Pubmed:9309578]
J Pept Res. 1997 Sep;50(3):153-8.
A series of Pro peptides containing the sequence of the oostatic hormone 3d and its shorter analogues 3a-3c differing in a number of the C-terminal Pro residues was prepared for a study of its effect on oogenesis in Sarcophaga bullata Parker (Diptera). Peptides 3a-3d were synthesized in solution by the fragment condensation of Boc-Tyr-Asp(OtBu)-Pro-Ala-Pro-OH (2f) with Pro oligopeptides H-(Pro)2-5-OtBu. The amino-terminal protected pentapeptide acid 2f was prepared by a stepwise procedure from TFA.H-Ala-Pro-OMe using Boc-Pro-OH, Z-Asp(OtBu)-OSu and Boc-Tyr-OSu. The H(Z)-(Pro)2-5-OtBu oligopeptides 1a-1h were synthesized from Z-Pro-OH and H-Pro-OtBu by a combination of stepwise procedure and fragment condensation. The 125I-labeled molecules of the octapeptide 3b and decapeptide 3d were used for radiotracer distribution studies. Evidence of content of the labeled peptide material in various parts of the insect body (ovaries, head, intestine) is presented. The time distribution of the labeled material in the insect organs was correlated with results of histological analysis of ovaries treated by nonlabeled peptides. The peptides assayed affected processes of egg development in 20-60% of ovarioles. The decapeptide 3d caused changes consisting in some resorbed egg chambers and normal appearance of vitellogenic eggs, whereas the octapeptide 3b caused abnormal yolk deposition and formation of big eggs with irregular yolk granules, proliferation of follicular epithelium in some egg chambers and about the same amount of resorbed egg chambers as decapeptide. These structural differences are complementary to the different values of organ radioactivities.


