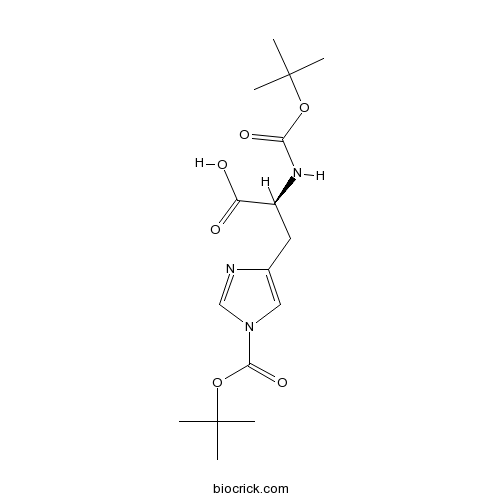
Boc-His(Boc)-OHCAS# 20866-46-0 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20866-46-0 | SDF | Download SDF |
| PubChem ID | 7023100 | Appearance | Powder |
| Formula | C16H25N3O6 | M.Wt | 355.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-3-[1-[(2-methylpropan-2-yl)oxycarbonyl]imidazol-4-yl]propanoic acid | ||
| SMILES | CC(C)(C)OC(=O)NC(CC1=CN(C=N1)C(=O)OC(C)(C)C)C(=O)O | ||
| Standard InChIKey | IXHPIPUIOSSAIS-NSHDSACASA-N | ||
| Standard InChI | InChI=1S/C16H25N3O6/c1-15(2,3)24-13(22)18-11(12(20)21)7-10-8-19(9-17-10)14(23)25-16(4,5)6/h8-9,11H,7H2,1-6H3,(H,18,22)(H,20,21)/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-His(Boc)-OH Dilution Calculator

Boc-His(Boc)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8137 mL | 14.0687 mL | 28.1373 mL | 56.2746 mL | 70.3433 mL |
| 5 mM | 0.5627 mL | 2.8137 mL | 5.6275 mL | 11.2549 mL | 14.0687 mL |
| 10 mM | 0.2814 mL | 1.4069 mL | 2.8137 mL | 5.6275 mL | 7.0343 mL |
| 50 mM | 0.0563 mL | 0.2814 mL | 0.5627 mL | 1.1255 mL | 1.4069 mL |
| 100 mM | 0.0281 mL | 0.1407 mL | 0.2814 mL | 0.5627 mL | 0.7034 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-His(Boc)-OH
- Berberine
Catalog No.:BCN4911
CAS No.:2086-83-1
- Primulic Acid 2
Catalog No.:BCC8237
CAS No.:208599-88-6
- H-Tle-OH
Catalog No.:BCC2659
CAS No.:20859-02-3
- Micafungin sodium
Catalog No.:BCC1750
CAS No.:208538-73-2
- Protoaescigenin
Catalog No.:BCC8240
CAS No.:20853-07-0
- Stigmastane-3,5,6-triol
Catalog No.:BCN4910
CAS No.:20835-91-0
- Ethyl 4-(rhamnosyloxy)benzylcarbamate
Catalog No.:BCN7635
CAS No.:208346-80-9
- Gentiopicroside
Catalog No.:BCN4909
CAS No.:20831-76-9
- Daunorubicin
Catalog No.:BCC4115
CAS No.:20830-81-3
- Digoxin
Catalog No.:BCN5359
CAS No.:20830-75-5
- ZM336372
Catalog No.:BCC3875
CAS No.:208260-29-1
- Zooxanthellabetaine A
Catalog No.:BCN1771
CAS No.:208256-89-7
- Ermanin
Catalog No.:BCN4912
CAS No.:20869-95-8
- (1R,1'S,3'R/1R,1'R,3'S)-L-054,264
Catalog No.:BCC7364
CAS No.:208706-12-1
- Saikosaponin D
Catalog No.:BCN1088
CAS No.:20874-52-6
- Testosterone benzoate
Catalog No.:BCC9166
CAS No.:2088-71-3
- Swertianin
Catalog No.:BCC8258
CAS No.:20882-75-1
- JWH 073
Catalog No.:BCC1674
CAS No.:208987-48-8
- Mangochinine
Catalog No.:BCN4913
CAS No.:209115-67-3
- Obtucarbamate B
Catalog No.:BCN3937
CAS No.:20913-18-2
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Fmoc- ß-HoAsp(OtBu)-OH
Catalog No.:BCC3230
CAS No.:209252-17-5
- Neocryptomerin
Catalog No.:BCN8023
CAS No.:20931-36-6
- Beta-mangostin
Catalog No.:BCN1213
CAS No.:20931-37-7
Synthesis of the novel pi-(benzyloxymethyl)-protected histidine analogue of statine. Inhibition of penicillopepsin by pepstatin-derived peptides containing different statine side-chain derivatives.[Pubmed:2661819]
J Med Chem. 1989 Jul;32(7):1571-6.
The synthesis of aspartic proteinase inhibitors derived from a new histidine side-chain analogue of statine (Sta), (3S,4S)-4-amino-3-hydroxy-5-(imidazol-4-yl)pentanoic acid (HiSta, 20), is reported. Boc-HiSta(BOM)-OMe (7) was prepared in 16% overall yield from Boc-His(pi-BOM)-OH via formation of the tetramic acid derivative 11 and stereoselective cis reduction with NaBH4 to the 4-hydroxy lactam 12. Removal of the Boc group from ester 7 (enantiomeric purity ee = 88-90%) and coupling to the tripeptide segment Iva-Val-Val-OH (13) by the DCC/HOBt preactivation method followed by hydrogenolytic removal of the pi-BOM group over Pd(OH)2 on carbon gave Iva-Val-Val-HiSta-OMe (16). This new peptide 16 is a very potent inhibitor of the fungal aspartic proteinase penicillopepsin (Ki = 4.5 x 10(-9) M) that is 10 times more active than the comparable Sta-containing inhibitor 3 and 2-3 times more potent than the new (3S,4S)-4-amino-3-hydroxy-5-phenylpentanoic acid (AHPPA) analogue 17 (Ki = 1.5 x 10(-8) M). However, compound 16, which has an imidazole residue at the P1 position, is a significantly weaker inhibitor of the enzyme than the corresponding analogues with the lysine (5) and ornithine (6) side chains at P1. Considerations that led to the synthesis of 16 and the results of the enzyme kinetics are discussed in detail.
X-ray analyses of aspartic proteinases. III Three-dimensional structure of endothiapepsin complexed with a transition-state isostere inhibitor of renin at 1.6 A resolution.[Pubmed:2266553]
J Mol Biol. 1990 Dec 20;216(4):1017-29.
The aspartic proteinase, endothiapepsin (EC 3.4.23.6), was complexed with a highly potent renin inhibitor, H-261 (t-Boc-His-Pro-Phe-His-LeuOHVal-Ile-His), where OH denotes a hydroxyethylene (-(S) CHOH-CH2-) transition-state isostere in the scissile bond surrogate. Crystals were grown in a form that has the same space group P2(1) as the uncomplexed enzyme, but with a 10 A decrease in the length of the alpha-axis and a 13 degrees decrease in the beta-angle. X-ray data have been collected to a resolution of 1.6 A. The rotation and translation parameters defining the position of the enzyme in the unit cell were determined previously using another enzyme-inhibitor complex that crystallized isomorphously with that of H-261. The molecule was refined using restrained least-squares refinement and the positions of non-hydrogen atoms of the inhibitor and water molecules were defined by difference Fourier techniques. The enzyme-inhibitor complex and 322 water molecules were further refined to a crystallographic R-factor of 0.14. Apart from a small rigid group rotation of a domain comprising residues 190 to 302 and small movements in the flap, there is little difference in conformation between the complexed and uncomplexed forms of the enzyme. The inhibitor is bound in an extended conformation along the active site cleft, and the hydroxyl group of the hydroxyethylene moiety is hydrogen-bonded to both catalytic aspartate carboxylates. The complex is stabilized by hydrogen bonds between the main-chain of the inhibitor and the enzyme. All side-chains of the inhibitor are in van der Waals' contact with groups in the enzyme and define a series of specificity pockets along the active site cleft. The study provides useful clues as to how this potent renin inhibitor (IC50 value of 0.7 x 10(-9) M) may bind renin. In particular it defines the interactions of the hydroxyethylene transition-state isostere with the enzyme more precisely than has been previously possible and therefore provides a useful insight into interactions in the transition state complex.
Thermal perturbation spectrophotometry of luliberin and model His-Trp peptides.[Pubmed:7030996]
Int J Pept Protein Res. 1981 Apr;17(4):460-8.
Thermal perturbation (TP) spectra of luliberin were measured at pH 5-8, and compared with the model chromophores N-Ac-Tyr-NH2, N-Ac-Trp-NH2, t-Boc-His-Trp-NH2, H-His-Trp-OH, Ac-His-Trp-OH, tryptophan and cyclo-[His-Trp] (all L-isomers). Between pH 5 and neutrality, the major TP extremum of the Trp3 residue of luliberin increases by about 50%. A similar effect is seen for luliberin acetylated on Tyr5. The effect with luliberin is attributed to the protonation of the His2 residue. One proposed explanation is that the protonated imidazole orients water around the nearby indole in a different way than does unprotonated imidazole. The Tyr5 residue of luliberin behaves like N-Ac-Tyr-NH2, and is considered to be well exposed to solvent. The TP spectra of N-Ac-Trp-NH2, t-Boc-His-Trp-NH2, Ac-His-Trp-OH, and cyclo-[His-Trp] are pH-independent from pH 5 to 8. The TP spectrum of H-His-Trp-NH2 has a bell-shaped pH dependence, rising from normal at pH 3.5 to above normal at pH 6, and returning to normal at pH 8. Luliberin and model peptides show that fluorescence and TP spectra of His-Trp sequences can respond differently to pH.
Potent in vivo inhibitors of rat renin: analogues of human and rat angiotensinogen sequences containing different classes of pseudodipeptides at the scissile site.[Pubmed:9352462]
J Pept Res. 1997 Oct;50(4):239-47.
Using solid-phase methodology we have synthesised peptides based on the 8-14 or 6-14 human and rat angiotensinogen sequences, containing the following different isosteric units at the P1-P1' cleavage site: Leu-psi[CH2NH]Leu; Leu-psi[CH(OH)CH2]Val; Leu-psi[CH(OH)CH2]Leu and Leu-psi[CH(NH2)CH2]Val. In vitro, peptide Piv-His-Pro-Phe-His-Leu-psi[CH(OH)CH2]Leu-Tyr-Tyr-Ser-NH2(XXI) is the most potent inhibitor of rat plasma renin reported having an IC50 of 0.21 nM; it is a much weaker inhibitor of human renin (IC50 45 nM). Peptide Boc-His-Pro-Phe-His-Leu-psi[CH(OH)CH2] Leu-Val-Ile-His-NH2 (XX) was a highly effective inhibitor of rat renin in vivo. When infused (1 mg/kg/h) into two-kidney, one-clip chronic renal hypertensive rats, it lowered blood pressure and suppressed both plasma renin and angiotensin II. When given as a bolus (1 mg/kg) there was a divergence between the rapid rebound of renin levels and blood pressure, which remained suppressed. These results indicate that potent in vivo inhibitors of rat renin could be useful not only in examining the role of circulating renin but also in elucidating the equally important involvement of extracirculatory renin pools.
[Synthesis of a fragment of the active center of the streptococcal proteinase (EC 3.4.22.10), VI (author's transl)].[Pubmed:130006]
Z Naturforsch C. 1975 Nov-Dec;30(6):739-44.
The synthesis and properties of the protected peptide Boc-His(Boc)-Cys(X)-Val-OH(ONP) [X = tert-butylmercapto, p-methoxybenzyl, tetrahydropyranyl-(2)] are described. This peptide is a fragment of an active center sequence of the streptococcal proteinase (EC 3.4.22.10) containing the essential SH group of the enzyme.


