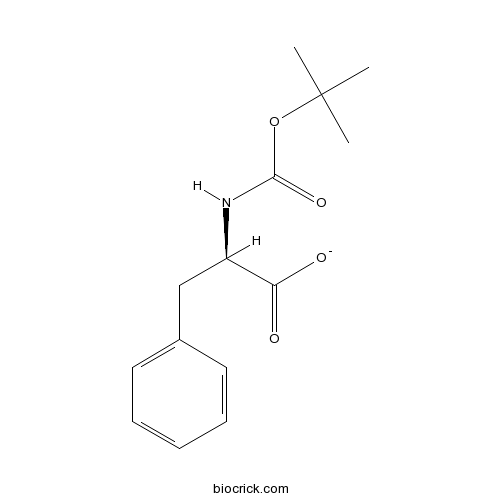
Boc-D-Phe-OHCAS# 18942-49-9 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18942-49-9 | SDF | Download SDF |
| PubChem ID | 1810766 | Appearance | Powder |
| Formula | C14H19NO4 | M.Wt | 265.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-3-phenylpropanoate | ||
| SMILES | CC(C)(C)OC(=O)NC(CC1=CC=CC=C1)C(=O)[O-] | ||
| Standard InChIKey | ZYJPUMXJBDHSIF-LLVKDONJSA-M | ||
| Standard InChI | InChI=1S/C14H19NO4/c1-14(2,3)19-13(18)15-11(12(16)17)9-10-7-5-4-6-8-10/h4-8,11H,9H2,1-3H3,(H,15,18)(H,16,17)/p-1/t11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-D-Phe-OH Dilution Calculator

Boc-D-Phe-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7693 mL | 18.8466 mL | 37.6932 mL | 75.3864 mL | 94.2329 mL |
| 5 mM | 0.7539 mL | 3.7693 mL | 7.5386 mL | 15.0773 mL | 18.8466 mL |
| 10 mM | 0.3769 mL | 1.8847 mL | 3.7693 mL | 7.5386 mL | 9.4233 mL |
| 50 mM | 0.0754 mL | 0.3769 mL | 0.7539 mL | 1.5077 mL | 1.8847 mL |
| 100 mM | 0.0377 mL | 0.1885 mL | 0.3769 mL | 0.7539 mL | 0.9423 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-D-Phe-OH
- Boc-Cys(pMeOBzl)-OH
Catalog No.:BCC3378
CAS No.:18942-46-6
- Chebulinic acid
Catalog No.:BCN3263
CAS No.:18942-26-2
- Triptinin B
Catalog No.:BCN6785
CAS No.:189389-05-7
- Endomorphin-1
Catalog No.:BCC1008
CAS No.:189388-22-5
- Corchoionol C
Catalog No.:BCN1172
CAS No.:189351-15-3
- Fmoc-Thr(tBu)-ol
Catalog No.:BCC2576
CAS No.:189337-28-8
- 3'-Methoxyrocaglamide
Catalog No.:BCN1171
CAS No.:189322-69-8
- 3'-Hydroxyrocaglamide
Catalog No.:BCN1170
CAS No.:189322-67-6
- Xanthohumol B
Catalog No.:BCN8018
CAS No.:189308-10-9
- Danshenol B
Catalog No.:BCN2616
CAS No.:189308-09-6
- Danshenol A
Catalog No.:BCN3145
CAS No.:189308-08-5
- N-Caffeoyl-O-methyltyramine
Catalog No.:BCC8216
CAS No.:189307-47-9
- Boc-Ser(tBu)-OH.DCHA
Catalog No.:BCC3445
CAS No.:18942-50-2
- Epothilone D
Catalog No.:BCC1554
CAS No.:189453-10-9
- Ginsenoside F5
Catalog No.:BCN6419
CAS No.:189513-26-6
- Pinostrobin chalcone
Catalog No.:BCN1173
CAS No.:18956-15-5
- 6'-Hydroxy-7'-ethoxybergamottin
Catalog No.:BCC8306
CAS No.:
- Dihydrooroxylin A
Catalog No.:BCN3500
CAS No.:18956-18-8
- trans-4-phenylbut-3-en-2-one
Catalog No.:BCN3805
CAS No.:1896-62-4
- 6,4'-Dihydroxy-7-methoxyflavanone
Catalog No.:BCN7797
CAS No.:189689-32-5
- Akuammiline
Catalog No.:BCN4772
CAS No.:1897-26-3
- Ro 10-5824 dihydrochloride
Catalog No.:BCC7330
CAS No.:189744-94-3
- 8-Amino-2-methylquinoline
Catalog No.:BCC8782
CAS No.:18978-78-4
- 3-O-Feruloylquinic acid
Catalog No.:BCN3353
CAS No.:1899-29-2
Molecularly Imprinted Polymers: Thermodynamic and Kinetic Considerations on the Specific Sorption and Molecular Recognition.[Pubmed:27879853]
Sensors (Basel). 2008 Apr 23;8(4):2854-2864.
This article presents a work aiming at thermodynamically and kinetically interpreting the specific sorption and recognition by a molecularly imprinted polymer. Using Boc-L-Phe-OH as a template, the imprinted material was prepared. The result indicates that the prepared polymer can well discriminate the imprint species from its analogue (Boc-D-Phe-OH), so as to adsorb more for the former but less for the latter. Kinetic analysis indicates that this specific sorption, in nature, can be a result of a preferential promotion. The imprint within the polymer causes a larger adsorption rate for the template than for the analogue. Thermodynamic study also implies that the molecular induction from the specific imprint to the template is larger than to the analogue, which thus makes the polymer capable of preferentially alluring the template to bind.


