BislineCAS# 30258-28-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

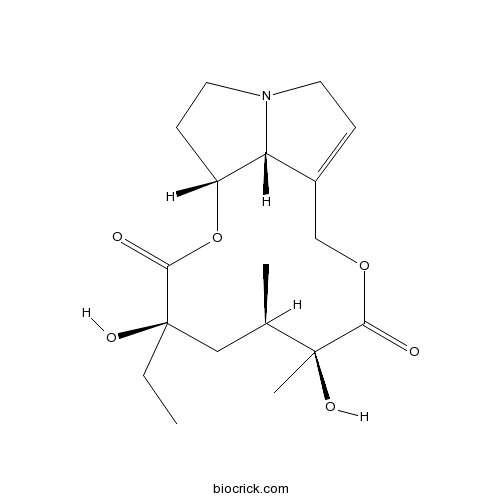
| Cas No. | 30258-28-7 | SDF | Download SDF |
| PubChem ID | 181990 | Appearance | Powder |
| Formula | C18H27NO6 | M.Wt | 353.41 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CCC1(CC(C(C(=O)OCC2=CCN3C2C(CC3)OC1=O)(C)O)C)O | ||
| Standard InChIKey | QZJRTVIGIAAJPX-GPBKISAOSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Simultaneous determination of isoline and its two major metabolites using high-performance liquid chromatography.[Pubmed: 14987418]J Anal Toxicol. 2004 Jan-Feb;28(1):11-5.
|
| Structure Identification | Phytochemistry. 2000 Aug;54(8):933-5.Structure revision of isoline (ruwenine), bisline and isolinecic acid.[Pubmed: 11014292]
Journal of the Chemical Society C Organic,1970 ,17 (17) :2312-2315.The Senecio alkaloids. Suggested structures for isoline and bisline, two new alkaloids from Senecio othonniformis Fourcade[Reference: WebLink]
|

Bisline Dilution Calculator

Bisline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8296 mL | 14.1479 mL | 28.2957 mL | 56.5915 mL | 70.7394 mL |
| 5 mM | 0.5659 mL | 2.8296 mL | 5.6591 mL | 11.3183 mL | 14.1479 mL |
| 10 mM | 0.283 mL | 1.4148 mL | 2.8296 mL | 5.6591 mL | 7.0739 mL |
| 50 mM | 0.0566 mL | 0.283 mL | 0.5659 mL | 1.1318 mL | 1.4148 mL |
| 100 mM | 0.0283 mL | 0.1415 mL | 0.283 mL | 0.5659 mL | 0.7074 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rosiglitazone HCl
Catalog No.:BCC2269
CAS No.:302543-62-0
- Potassium 7-hydroxynaphthalene-1-sulfonate
Catalog No.:BCN8289
CAS No.:30252-40-5
- 1-Hydroxybaccatin I
Catalog No.:BCN5211
CAS No.:30244-37-2
- Ingenol
Catalog No.:BCN2333
CAS No.:30220-46-3
- Effusanin A
Catalog No.:BCN5210
CAS No.:30220-43-0
- Arenobufagin 3-hemisuberate
Catalog No.:BCN7837
CAS No.:30219-16-0
- Retinoic acid
Catalog No.:BCN2185
CAS No.:302-79-4
- DL-Alanine
Catalog No.:BCN8539
CAS No.:302-72-7
- Aconitine
Catalog No.:BCN1014
CAS No.:302-27-2
- Hydroxyprogesterone acetate
Catalog No.:BCC8997
CAS No.:302-23-8
- Desoxyrhaponticin
Catalog No.:BCN2954
CAS No.:30197-14-9
- Ro 5-3335
Catalog No.:BCC7962
CAS No.:30195-30-3
- 3,4,5-Trimethoxycinnamyl alcohol
Catalog No.:BCN5212
CAS No.:30273-62-2
- TCS 46b
Catalog No.:BCC7482
CAS No.:302799-86-6
- Ciliobrevin A
Catalog No.:BCC3939
CAS No.:302803-72-1
- Ro 01-6128
Catalog No.:BCC7922
CAS No.:302841-86-7
- Ro 67-4853
Catalog No.:BCC7921
CAS No.:302841-89-0
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- 2-Amino-N-(2-chloro-6-methylphenyl) thiazole-5-carboxamide
Catalog No.:BCC8551
CAS No.:302964-24-5
- Clinofibrate
Catalog No.:BCC5020
CAS No.:30299-08-2
- Heliotrine
Catalog No.:BCN1982
CAS No.:303-33-3
- Lasiocarpine
Catalog No.:BCN2001
CAS No.:303-34-4
- Methenolone enanthate
Catalog No.:BCC9029
CAS No.:303-42-4
- Gossypol
Catalog No.:BCN2702
CAS No.:303-45-7
Simultaneous determination of isoline and its two major metabolites using high-performance liquid chromatography.[Pubmed:14987418]
J Anal Toxicol. 2004 Jan-Feb;28(1):11-5.
A simple and reliable high-performance liquid chromatographic (HPLC) assay was developed for a simultaneous determination of isoline, a potent hepatotoxic pyrrolizidine alkaloid, and its two major metabolites, namely M1 (Bisline) and M2 (Bisline lactone, a new pyrrolizidine alkaloid). The latter two metabolites were produced during in vitro metabolism of isoline by rat and mouse microsomal enzyme systems. The analysis was conducted by a direct injection of aliquots of supernatant of the microsomal reaction mixture treated with the equal volume of ice-cold methanol onto a conventional reversed-phase analytical column (150 x 4.6 mm). The analytes were separated by a gradient elution with mobile phases A (0.01 M dihydro-potassium phosphate, pH 4.8) and B (acetonitrile). The assay has shown excellent precision and accuracy with less than 10% of overall intra- and interday variations and higher than 94% of overall accuracy. The developed HPLC method was successfully applied for the determination of the intact isoline and its two pH- and thermally labile metabolites produced in rat and mouse liver microsomal incubations.
Structure revision of isoline (ruwenine), bisline and isolinecic acid.[Pubmed:11014292]
Phytochemistry. 2000 Aug;54(8):933-5.
X-ray crystallography of Bisline, and the chemical interconversion of Bisline and isoline (ruwenine), revealed that the structures previously assigned to these alkaloids required revision; as did that of isolinecic acid.


