BerberrubineCAS# 15401-69-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15401-69-1 | SDF | Download SDF |
| PubChem ID | 72703 | Appearance | Yellow-orange powder |
| Formula | C19H16NO4+ | M.Wt | 322.33 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | 9-Berberoline chloride; Beroline chloride; 9-Demethoxy 9-hydroxyberberinium chloride | ||
| Solubility | Soluble in methan | ||
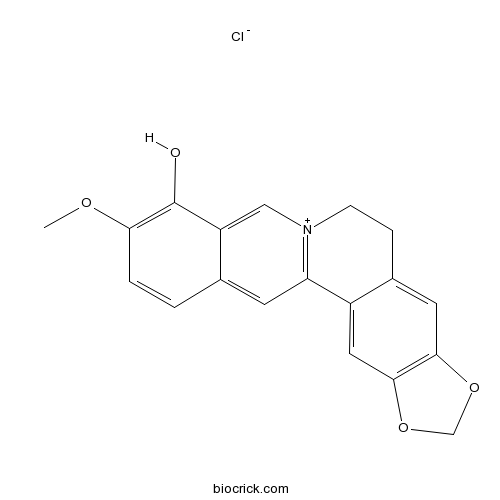
| SMILES | COC1=C(C2=C[N+]3=C(C=C2C=C1)C4=CC5=C(C=C4CC3)OCO5)O.[Cl-] | ||
| Standard InChIKey | GYFSYEVKFOOLFZ-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Berberrubine possesses diverse pharmacological activities, including glucose-lowering, lipid-lowering, anti-inflammatory, and anti-tumor effects. Berberrubine dose-dependently inhibits IL-8 and MCP-1 protein levels in the media and mRNA expression of the cells stimulated with IL-1beta or TNF-alpha. |
| Targets | LDL | IL Receptor | TNF-α | NF-kB | MCP-1 |
| In vitro | Effect of berberrubine on interleukin-8 and monocyte chemotactic protein-1 expression in human retinal pigment epithelial cell line.[Pubmed: 16797033]Life Sci. 2006 Aug 1;79(10):949-56.We examined the effects of Berberrubine, a protoberberine alkaloid, on interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1) expression in a human retinal pigment epithelial cell line (ARPE-19) stimulated with interleukin-1beta (IL-1beta) or tumor necrosis factor alpha (TNF-alpha). |
| In vivo | Design, synthesis, and cholesterol-lowering efficacy for prodrugs of berberrubine.[Pubmed: 20673726]Bioorg Med Chem. 2010 Sep 1;18(17):6422-8.
|
| Cell Research | Antitumor activity of berberrubine derivatives.[Pubmed: 964560]Gan. 1976 Apr;67(2):321-5.
|
| Structure Identification | Bioorg Med Chem Lett. 2014 Apr 1;24(7):1762-5.Synthesis and in vitro evaluation of 12-(substituted aminomethyl) berberrubine derivatives as anti-diabetics.[Pubmed: 24613165]
|

Berberrubine Dilution Calculator

Berberrubine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1024 mL | 15.5121 mL | 31.0241 mL | 62.0482 mL | 77.5603 mL |
| 5 mM | 0.6205 mL | 3.1024 mL | 6.2048 mL | 12.4096 mL | 15.5121 mL |
| 10 mM | 0.3102 mL | 1.5512 mL | 3.1024 mL | 6.2048 mL | 7.756 mL |
| 50 mM | 0.062 mL | 0.3102 mL | 0.6205 mL | 1.241 mL | 1.5512 mL |
| 100 mM | 0.031 mL | 0.1551 mL | 0.3102 mL | 0.6205 mL | 0.7756 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ipecoside
Catalog No.:BCN8301
CAS No.:15401-60-2
- Carmustine
Catalog No.:BCC5244
CAS No.:154-93-8
- Bz-Arg-OH
Catalog No.:BCC2856
CAS No.:154-92-7
- Tripelennamine HCl
Catalog No.:BCC4523
CAS No.:154-69-8
- Primulaverin
Catalog No.:BCC8235
CAS No.:154-61-0
- Primverin
Catalog No.:BCC8238
CAS No.:154-60-9
- 1,5-Anhydro-D-glucitol
Catalog No.:BCN2234
CAS No.:154-58-5
- Thioguanine
Catalog No.:BCC2220
CAS No.:154-42-7
- Catechin
Catalog No.:BCN1688
CAS No.:154-23-4
- Lincomycin
Catalog No.:BCC9010
CAS No.:154-21-2
- 2-Deoxy-D-glucose
Catalog No.:BCC4048
CAS No.:154-17-6
- ANQ 11125
Catalog No.:BCC6359
CAS No.:153966-48-4
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- Fuscaxanthone C
Catalog No.:BCN3885
CAS No.:15404-76-9
- Isonormangostin
Catalog No.:BCN1687
CAS No.:15404-80-5
- 3-(Bromomethyl)-2-cyclopropyl-4-(4'-fluorophenyl)quinoline
Catalog No.:BCC8591
CAS No.:154057-56-4
- ((2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)methyl)triphenylphosphonium bromide
Catalog No.:BCC8373
CAS No.:154057-58-6
- 2-(3-Methoxypropyl)-4-oxo-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8480
CAS No.:154127-41-0
- 4-Hydroxy-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8707
CAS No.:154127-42-1
- PD 144418 oxalate
Catalog No.:BCC7429
CAS No.:154130-99-1
- DMAB-anabaseine dihydrochloride
Catalog No.:BCC7301
CAS No.:154149-38-9
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
Effect of berberrubine on interleukin-8 and monocyte chemotactic protein-1 expression in human retinal pigment epithelial cell line.[Pubmed:16797033]
Life Sci. 2006 Aug 1;79(10):949-56.
We examined the effects of Berberrubine, a protoberberine alkaloid, on interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1) expression in a human retinal pigment epithelial cell line (ARPE-19) stimulated with interleukin-1beta (IL-1beta) or tumor necrosis factor alpha (TNF-alpha). ARPE-19 cells were cultured to confluence. Berberrubine and IL-1beta or TNF-alpha were added to the medium. IL-8 and MCP-1 protein concentrations were measured using enzyme-linked immunosorbent assay. IL-8 and MCP-1 mRNA were measured by real time polymerase chain reaction. Nuclear factor kappaB (NF-kappaB) translocation was examined by immunofluorescent staining/microscopy. Berberrubine dose-dependently inhibited IL-8 and MCP-1 protein levels in the media and mRNA expression of the cells stimulated with IL-1beta or TNF-alpha. Immunofluorescent staining/microscopy of NF-kappaB in the nucleus of unstimulated cells was faint (51+/-14 arbitrary units). Fluorescein was dense (215+/-42 or 170+/-24 arbitrary units, respectively) 30 min after stimulation with IL-1beta or TNF-alpha and was decreased to 62+/-18 or 47+/-16 arbitrary units, respectively, by Berberrubine. Berberrubine dose-dependently inhibited IL-8 and MCP-1 expression and protein secretion induced by IL-1beta or TNF-alpha. Possibly, the effect on chemotactic factors may be via suppression of NF-kappaB translocation.
Antitumor activity of berberrubine derivatives.[Pubmed:964560]
Gan. 1976 Apr;67(2):321-5.
The antitumor activity of berberine, Berberrubine, and their derivatives against sarcoma-180 ascites was determined by the total packed cell volume method. Berbine and tetrahydroberberine derivatives had no antitumor activity, but Berberrubine (9-demethylberberine) and the ester derivatives of Berberrubine had a strong antitumor activity. ED90 of Berberrubine, its acetate and benzoate, were 15, 23, and 44 mg/kg, respectively. The therapeutic indices (LD10/ED90 by the present method) of these compounds were as follows: Berberrubine hydrochloride, 6.7 approximately 9.4; 9-acetyl-9-demethylberberine (9-acetylBerberrubine) chloride, 7.6 approximately 8.7; 9-benzoyl-9-demethylberberine (9-benzoylBerberrubine) chloride, 3.4 approximately 4.9.
Design, synthesis, and cholesterol-lowering efficacy for prodrugs of berberrubine.[Pubmed:20673726]
Bioorg Med Chem. 2010 Sep 1;18(17):6422-8.
In order to enhance oral bioavailability of berberine (BBR) for its cholesterol-lowering efficacy in vivo, a series of ester or ether prodrugs of Berberrubine (M1), which is an active metabolite of BBR after first-pass metabolism, were designed, semi-synthesized, and evaluated. Among these M1 prodrugs, compound 5g possessing palmitate at the 9-position showed a moderate LogP value and esterase hydrolysis rate for releasing M1 in blood. Its cholesterol-lowering efficacy in vivo was evaluated in hyperlipidemic SD rats. Compound 5g (100mg/kg/d) reduced blood CHO and LDL-c by 35.8% and 45.5%, respectively, similar to that by BBR. It also exhibited a good safety in rats with no side-effect on liver and kidney function. Therefore, the design of M1 prodrug appears to be an effective strategy to improve pharmacokinetic feature of BBR for its lipid-lowering efficacy in vivo.
Synthesis and in vitro evaluation of 12-(substituted aminomethyl) berberrubine derivatives as anti-diabetics.[Pubmed:24613165]
Bioorg Med Chem Lett. 2014 Apr 1;24(7):1762-5.
By introducing various amino methyl groups into 12-position of Berberrubine, a series of 12-(substituted aminomethyl) Berberrubine derivatives were synthesized and evaluated for their anti-diabetic activity against type 2 diabetes mellitus. The results indicated that most of the prepared compounds exhibited moderate to good anti-diabetic activity, which were comparable to or even better than the berberine, the positive control rosiglitazone and insulin. Especially, compound 3b with an N-methyl piperazine-4-methyl group at C-12, exerted the most powerful anti-diabetic activity.


