ArvanilPotent TRPV1 (VR1) and CB1 agonist/anandamide transport inhibitor CAS# 128007-31-8 |
- Coptisine sulfate
Catalog No.:BCN2286
CAS No.:1198398-71-8
- Procyanidin B2
Catalog No.:BCN6315
CAS No.:29106-49-8
- Onjisaponin B
Catalog No.:BCN2741
CAS No.:35906-36-6
- Gypenoside XLIX
Catalog No.:BCN1207
CAS No.:94987-08-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 128007-31-8 | SDF | Download SDF |
| PubChem ID | 6449767 | Appearance | Powder |
| Formula | C28H41NO3 | M.Wt | 439.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in ethanol (supplied pre-dissolved in anhydrous ethanol, 5mg/ml) | ||
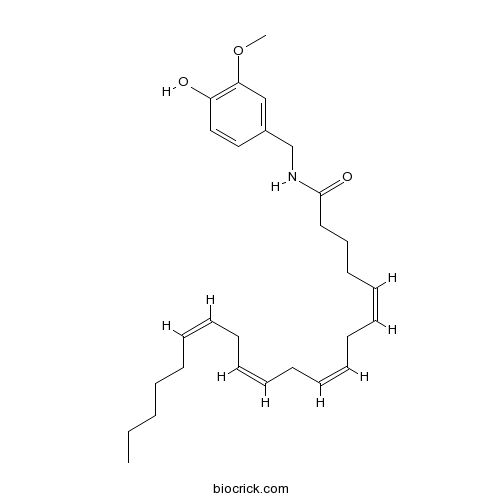
| Chemical Name | (5Z,8Z,11Z,14Z)-N-[(4-hydroxy-3-methoxyphenyl)methyl]icosa-5,8,11,14-tetraenamide | ||
| SMILES | CCCCCC=CCC=CCC=CCC=CCCCC(=O)NCC1=CC(=C(C=C1)O)OC | ||
| Standard InChIKey | QVLMCRFQGHWOPM-ZKWNWVNESA-N | ||
| Standard InChI | InChI=1S/C28H41NO3/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-28(31)29-24-25-21-22-26(30)27(23-25)32-2/h7-8,10-11,13-14,16-17,21-23,30H,3-6,9,12,15,18-20,24H2,1-2H3,(H,29,31)/b8-7-,11-10-,14-13-,17-16- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cannabinoid CB1 and vanilloid TRPV1 (VR1) agonist (Ki values are 0.5 and 0.3 μM respectively). Also inhibits the anandamide transporter (IC50 = 3.6 μM). Analgesic, vasodilatory and anti-inflammatory in vivo. |

Arvanil Dilution Calculator

Arvanil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2746 mL | 11.3729 mL | 22.7459 mL | 45.4918 mL | 56.8647 mL |
| 5 mM | 0.4549 mL | 2.2746 mL | 4.5492 mL | 9.0984 mL | 11.3729 mL |
| 10 mM | 0.2275 mL | 1.1373 mL | 2.2746 mL | 4.5492 mL | 5.6865 mL |
| 50 mM | 0.0455 mL | 0.2275 mL | 0.4549 mL | 0.9098 mL | 1.1373 mL |
| 100 mM | 0.0227 mL | 0.1137 mL | 0.2275 mL | 0.4549 mL | 0.5686 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sennoside B
Catalog No.:BCN1003
CAS No.:128-57-4
- Pregnanolone
Catalog No.:BCC7736
CAS No.:128-20-1
- Ursodiol
Catalog No.:BCC4945
CAS No.:128-13-2
- Teucrin A
Catalog No.:BCC8259
CAS No.:12798-51-5
- CU CPT 4a
Catalog No.:BCC6319
CAS No.:1279713-77-7
- 24(31)-Dehydrocarboxyacetylquercinic acid
Catalog No.:BCN1589
CAS No.:127970-62-1
- CGP 39551
Catalog No.:BCC7053
CAS No.:127910-32-1
- CGP 37849
Catalog No.:BCC7078
CAS No.:127910-31-0
- C-type natriuretic peptide (1-22) (human, rat, swine)
Catalog No.:BCC6033
CAS No.:127869-51-6
- Saquinavir
Catalog No.:BCC1921
CAS No.:127779-20-8
- Dryocrassin ABBA
Catalog No.:BCN6276
CAS No.:12777-70-7
- 2'-O-Methylbroussonin A
Catalog No.:BCN7318
CAS No.:127757-13-5
- erythro-1-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCN1588
CAS No.:1280602-81-4
- Fmoc-D-Ser(tBu)-OH
Catalog No.:BCC3548
CAS No.:128107-47-1
- Escitalopram
Catalog No.:BCC4193
CAS No.:128196-01-0
- N-ArachidonylGABA
Catalog No.:BCC7186
CAS No.:128201-89-8
- (R,R)-2,6-Bis(4-phenyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8397
CAS No.:128249-70-7
- GDC-0032
Catalog No.:BCC4066
CAS No.:1282512-48-4
- BAY-X 1005
Catalog No.:BCC6038
CAS No.:128253-31-6
- Pachyaximine A
Catalog No.:BCN6152
CAS No.:128255-08-3
- Axillaridine A
Catalog No.:BCN6153
CAS No.:128255-16-3
- 1-(3,4-Dimethoxycinnamoyl)piperidine
Catalog No.:BCN4036
CAS No.:128261-84-7
- Bivalirudin Trifluoroacetate
Catalog No.:BCC1421
CAS No.:128270-60-0
- 2alpha-Hydroxy-8beta-(2-methylbutyryloxy)costunolide
Catalog No.:BCN7319
CAS No.:128286-87-3
Non-pungent long chain capsaicin-analogs arvanil and olvanil display better anti-invasive activity than capsaicin in human small cell lung cancers.[Pubmed:27196129]
Cell Adh Migr. 2017 Jan 2;11(1):80-97.
The nutritional compound capsaicin inhibits the invasion of many types of human cancers. The clinical development of capsaicin as an anti-cancer drug is limited due to its unfavorable side effects like burning sensation, stomach cramps, gut pain and nausea. This study compared the anti-invasive activity of capsaicin to non-pungent long chain capsaicin analogs, namely Arvanil and olvanil, in human small cell lung cancer cells. Boyden chamber invasion assays revealed that Arvanil and olvanil displayed improved anti-invasive activity relative to capsaicin in human SCLC cells. The results of the Boyden chamber assay were confirmed by the spherical invasion assay, and similar results were obtained. The anti-invasive activity of Arvanil, olvanil and capsaicin were independent of TRPV and CB1 receptors. Furthermore, the anti-invasive activity of Arvanil, olvanil and capsaicin was mediated by the AMPK pathway. Depletion of AMPK levels by siRNA methodology abrogated the anti-invasive activity of Arvanil, olvanil and capsaicin. The non-pungent capsaicin analogs Arvanil and olvanil display improved anti-invasive activity relative to capsaicin in human SCLC cells. These agents may represent the second generation of capsaicin-like compounds which are more potent than the parent molecule and have a better side effect profile.
Role of VR1 and CB1 receptors in modelling of cardio-respiratory response to arvanil, an endocannabinoid and vanilloid hybrid, in rats.[Pubmed:18572197]
Life Sci. 2008 Jul 18;83(3-4):85-91.
Cardio-respiratory effects of an intravenous injection of Arvanil, a structural "hybrid" between capsaicin and anandamide, were investigated in 40 urethane-chloralose anaesthetized and spontaneously breathing rats. In the group of rats the response to Arvanil was checked to establish the appropriate dose of the drug. To analyze the pattern of the cardio-respiratory effects rats were challenged with bolus injection of Arvanil (0.8 mg kg(-1)) into the femoral vein. Administration of the drug evoked, in all tested rats, a significant increase of tidal volume (V(T)) and diaphragm activity, hypertension coupled with a fall in respiratory rate (f). To test the contribution of vanilloid (VR1) and cannabinoid (CB1) receptors to post-Arvanil response, administrations of the drug were preceded by nonselective VR1 antagonist ruthenium red, selective VR1 antagonist SB366791 or selective CB1 antagonist AM281. All antagonists eliminated an increase in V(T) but failed to block the hypertension evoked by Arvanil. Ruthenium red as well as SB366791 abolished post-Arvanil fall in respiratory rate. The rise of diaphragm activity was totally eliminated by ruthenium red and markedly reduced by SB366791. AM281 blockade of post-Arvanil changes in f and diaphragm activity was ineffective. These findings indicated that the post-Arvanil rise of V(T) was mediated by both VR1 and CB1 receptors. Only vanilloid receptors were involved in the increase of diaphragm activity and decrease of respiratory frequency. Hypertensive response to Arvanil might depend on different types of receptors.
Midcervical vagotomy precludes respiratory response to novel anti-inflammatory and anti-tumour drug arvanil in rats.[Pubmed:20599930]
Eur J Pharmacol. 2010 Sep 15;643(1):101-6.
Arvanil is a metabolically stable hybrid between anandamide and capsaicin. The present study was designed to test the role of the vagal pathway in post-Arvanil respiratory and blood pressure responses. Respiratory and pressure changes evoked by an intravenous injection of Arvanil were investigated in 21 urethane-chloralose anaesthetised and spontaneously breathing rats. In control neurally intact rats the effects of Arvanil were checked to establish the appropriate dose of the drug. In the experimental group rats were challenged with Arvanil while intact, following bilateral midcervical vagotomy and after subsequent supranodose vagotomy. In all neurally intact animals bolus injection of 0.8 mg/kg of Arvanil into the right femoral vein induced a significant increase of tidal volume (+1+/-0.11 ml; P<0.01) and diaphragm activity (+1.72+/-0.1 arbitrary units; P<0.01) as well as hypertension (+31.9+/-2.9 mm Hg; P<0.001) and a fall in respiratory rate (-24.7+/-0.4 breath/min; P<0.001). Bilateral midcervical vagotomy precluded the alteration of respiratory parameters but did not eliminate blood pressure response. Arvanil-induced increase in mean arterial blood pressure still persisted after supranodose vagotomy. Results indicated that the respiratory effects evoked by Arvanil administered via the peripheral circulation require intact midcervical vagi. Supranodose vagotomy failed to eliminate the hypertension evoked by Arvanil.
Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret.[Pubmed:17459108]
Eur J Neurosci. 2007 May;25(9):2773-82.
Cannabinoid (CB) agonists suppress nausea and vomiting (emesis). Similarly, transient receptor potential vanilloid-1 (TRPV1) receptor agonists are anti-emetic. Arvanil, N-(3-methoxy-4-hydroxy-benzyl)-arachidonamide, is a synthetic 'hybrid' agonist of CB1 and TRPV1 receptors. Anandamide and N-arachidonoyl-dopamine (NADA) are endogenous agonists at both these receptors. We investigated if Arvanil, NADA and anandamide were anti-emetic in the ferret and their mechanism of action. All compounds reduced the episodes of emesis in response to morphine 6 glucuronide. These effects were attenuated by AM251, a CB1 antagonist that was pro-emetic per se, and TRPV1 antagonists iodoresiniferatoxin and AMG 9810, which were without pro-emetic effects. Similar sensitivity to Arvanil and NADA was found for prodromal signs of emesis. We analysed the distribution of TRPV1 receptors in the ferret brainstem and, for comparison, the co-localization of CB1 and TRPV1 receptors in the mouse brainstem. TRPV1 immunoreactivity was largely restricted to the nucleus of the solitary tract of the ferret, with faint labeling in the dorsal motor nucleus of the vagus and sparse distribution in the area postrema. A similar distribution of TRPV1, and its extensive co-localization with CB1, was observed in the mouse. Our findings suggest that CB1 and TRPV1 receptors in the brainstem play a major role in the control of emesis by agonists of these two receptors. While there appears to be an endogenous 'tone' of CB1 receptors inhibiting emesis, this does not seem to be the case for TRPV1 receptors, indicating that endogenously released endocannabinoids/endovanilloids inhibit emesis preferentially via CB1 receptors.
Neurobehavioral activity in mice of N-vanillyl-arachidonyl-amide.[Pubmed:11040343]
Eur J Pharmacol. 2000 Oct 20;406(3):363-74.
We studied the cannabimimetic properties of N-vanillyl-arachidonoyl-amide (Arvanil), a potential agonist of cannabinoid CB(1) and capsaicin VR(1) receptors, and an inhibitor of the facilitated transport of the endocannabinoid anandamide. Arvanil and anandamide exhibited similar affinities for the cannabinoid CB(1) receptor, but Arvanil was less efficacious in inducing cannabinoid CB(1) receptor-mediated GTPgammaS binding. The K(i) of Arvanil for the vanilloid VR(1) receptor was 0.28 microM. Administered i.v. to mice, Arvanil was 100 times more potent than anandamide in producing hypothermia, analgesia, catalepsy and inhibiting spontaneous activity. These effects were not attenuated by the cannabinoid CB(1) receptor antagonist N-(piperidin-1-yl)-5-(4-chloro-phenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.HCl (SR141716A). Arvanil (i.t. administration) induced analgesia in the tail-flick test that was not blocked by either SR141716A or the vanilloid VR(1) antagonist capsazepine. Conversely, capsaicin was less potent as an analgesic (ED(50) 180 ng/mouse, i.t.) and its effects attenuated by capsazepine. The analgesic effect of anandamide (i.t.) was also unaffected by SR141716A but was 750-fold less potent (ED(50) 20.5 microg/mouse) than capsaicin. These data indicate that the neurobehavioral effects exerted by Arvanil are not due to activation of cannabinoid CB(1) or vanilloid VR(1) receptors.
New perspectives on enigmatic vanilloid receptors.[Pubmed:11006466]
Trends Neurosci. 2000 Oct;23(10):491-7.
In spite of the rapid advances in our understanding of vanilloid-receptor pharmacology in the PNS, the function of vanilloid receptors in the brain has remained elusive. Recently, the endocannabinoid anandamide has been proposed to function as an endogenous agonist at the vanilloid receptor VR1. This is an exciting hypothesis because the localization of VR1 overlaps with that of anandamide and its preferred cannabinoid receptor CB(1) in various brain areas. The interaction of anandamide and/or related lipid metabolites with these two completely separate receptor systems in the brain clearly places VR1 in a much broader role than pain perception. At a practical level, the overlapping ligand recognition properties of VR1 and CB(1) might be exploited by medicinal chemistry. For example, Arvanil, a 'chimeric' ligand that combines structural features of capsaicin and anandamide, promises to be an interesting lead for new drugs that interact at both vanilloid and cannabinoid receptors.
Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors.[Pubmed:10448105]
Biochem Biophys Res Commun. 1999 Aug 19;262(1):275-84.
We investigated the effect of changing the length and degree of unsaturation of the fatty acyl chain of N-(3-methoxy-4-hydroxy)-benzyl-cis-9-octadecenoamide (olvanil), a ligand of vanilloid receptors, on its capability to: (i) inhibit anandamide-facilitated transport into cells and enzymatic hydrolysis, (ii) bind to CB1 and CB2 cannabinoid receptors, and (iii) activate the VR1 vanilloid receptor. Potent inhibition of [(14)C]anandamide accumulation into cells was achieved with C20:4 n-6, C18:3 n-6 and n-3, and C18:2 n-6 N-acyl-vanillyl-amides (N-AVAMs). The saturated analogues and Delta(9)-trans-olvanil were inactive. Activity in CB1 binding assays increased when increasing the number of cis-double bonds in a n-6 fatty acyl chain and, in saturated N-AVAMs, was not greatly sensitive to decreasing the chain length. The C20:4 n-6 analogue (Arvanil) was a potent inhibitor of anandamide accumulation (IC(50) = 3.6 microM) and was 4-fold more potent than anandamide on CB1 receptors (Ki = 0.25-0.52 microM), whereas the C18:3 n-3 N-AVAM was more selective than Arvanil for the uptake (IC(50) = 8.0 microM) vs CB1 receptors (Ki = 3.4 microM). None of the compounds efficiently inhibited [(14)C]anandamide hydrolysis or bound to CB2 receptors. All N-AVAMs activated the cation currents coupled to VR1 receptors overexpressed in Xenopus oocytes. In a simple, intact cell model of both vanilloid- and anandamide-like activity, i.e., the inhibition of human breast cancer cell (HBCC) proliferation, Arvanil was shown to behave as a "hybrid" activator of cannabinoid and vanilloid receptors.


