AromadendreneCAS# 489-39-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 489-39-4 | SDF | Download SDF |
| PubChem ID | 11095734 | Appearance | Oil |
| Formula | C15H24 | M.Wt | 204.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
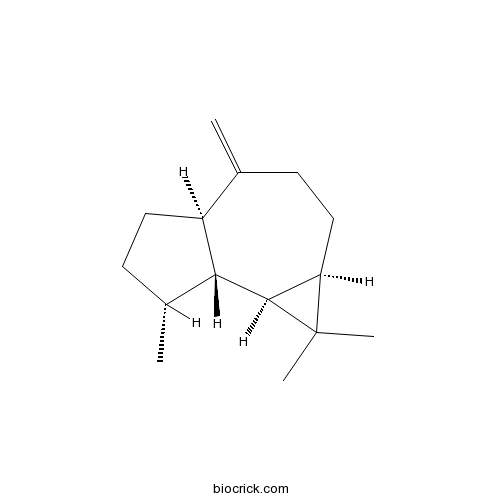
| Chemical Name | (1aR,4aR,7R,7aR,7bS)-1,1,7-trimethyl-4-methylidene-2,3,4a,5,6,7,7a,7b-octahydro-1aH-cyclopropa[e]azulene | ||
| SMILES | CC1CCC2C1C3C(C3(C)C)CCC2=C | ||
| Standard InChIKey | ITYNGVSTWVVPIC-XVIXHAIJSA-N | ||
| Standard InChI | InChI=1S/C15H24/c1-9-6-8-12-14(15(12,3)4)13-10(2)5-7-11(9)13/h10-14H,1,5-8H2,2-4H3/t10-,11+,12-,13-,14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Aromadendrene has significant antibacterial activity against the Gram-positive and Gram-negative bacteria. |
| Targets | Antifection |

Aromadendrene Dilution Calculator

Aromadendrene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8924 mL | 24.4618 mL | 48.9237 mL | 97.8474 mL | 122.3092 mL |
| 5 mM | 0.9785 mL | 4.8924 mL | 9.7847 mL | 19.5695 mL | 24.4618 mL |
| 10 mM | 0.4892 mL | 2.4462 mL | 4.8924 mL | 9.7847 mL | 12.2309 mL |
| 50 mM | 0.0978 mL | 0.4892 mL | 0.9785 mL | 1.9569 mL | 2.4462 mL |
| 100 mM | 0.0489 mL | 0.2446 mL | 0.4892 mL | 0.9785 mL | 1.2231 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Angelicide
Catalog No.:BCN8662
CAS No.:92935-94-9
- Glicoricone
Catalog No.:BCN8661
CAS No.:161099-37-2
- Bacopaside I
Catalog No.:BCN8660
CAS No.:382148-47-2
- Vinaginsenoside R4
Catalog No.:BCN8659
CAS No.:156009-80-2
- Nuezhenidic acid
Catalog No.:BCN8658
CAS No.:183238-67-7
- Kanzonol C
Catalog No.:BCN8657
CAS No.:151135-82-9
- Ginsenoside Rh7
Catalog No.:BCN8656
CAS No.:343780-68-7
- 5-Ethoxy-10-Gingerol
Catalog No.:BCN8655
CAS No.:121771-98-0
- Angelicolide
Catalog No.:BCN8654
CAS No.:90826-58-7
- 11alpha-Methoxysaikosaponin F
Catalog No.:BCN8653
CAS No.:104109-37-7
- Daidzein diacetate
Catalog No.:BCN8652
CAS No.:3682-01-7
- 1-Methyl-6-oxopyridine-3-carboxylic acid
Catalog No.:BCN8651
CAS No.:3719-45-7
- Parvisoflavanone
Catalog No.:BCN8664
CAS No.:49776-79-6
- Lappaol F
Catalog No.:BCN8665
CAS No.:69394-17-8
- 4''-methyloxy-Daidzin
Catalog No.:BCN8667
CAS No.:1195968-02-5
- 4''-methyloxy-Genistin
Catalog No.:BCN8668
CAS No.:950910-16-4
- Ophiopogonanone B
Catalog No.:BCN8669
CAS No.:1316759-83-7
- Moscatin
Catalog No.:BCN8670
CAS No.:108335-06-4
- Epimagnolin B
Catalog No.:BCN8671
CAS No.:1134188-26-3
- Resveratroloside
Catalog No.:BCN8672
CAS No.:38963-95-0
- Pomiferin
Catalog No.:BCN8673
CAS No.:572-03-2
- Aurantio-obtusin beta-D-glucoside
Catalog No.:BCN8674
CAS No.:129025-96-3
- 13-Methylberberine
Catalog No.:BCN8675
CAS No.:54260-72-9
- Lappaol C
Catalog No.:BCN8676
CAS No.:64855-00-1
Effect of simultaneous snail slime-aided degradation and yeast fermentation on terpenoid composition of plantain pseudostem waste.[Pubmed:30961480]
Curr Pharm Biotechnol. 2019 Apr 8. pii: CPB-EPUB-97888.
BACKGROUND: In this study, local sustainable enzyme sources involving excised digestive juice of African land snail and yeast were to achieve the simultaneous saccharification and fermentation (SSF) of plantain pseudo stem (PPS) waste, and afterwards examined their effects on terpenoids using gas chromatograpy coupled to a fluorescent ionization detector (GC-FID). RESULTS: The most abundant terpenoids were found in the order alpha-pinene > borneol > camphor > humulene > beta-caryophellene, while the least in abundance were cis ocimene (8.78x10-6 mg/100g), and cyperene (1.81x10-5 mg/100g). The application of exclusive fermentation, and SSF respectively elevated azuluene by 95.46 and 99.6 %, while pinene-2-ol was elevated by 83.02 and 98.57 % respectively. Both exclusive fermentation and SSF had no effect on myrcene, cyperene, ethyl cinnamate, germacrene b, valencene, beta selinene, Aromadendrene, and taraxerol, while the degree of degradation of some of the terpenoids by both processes were respectively as follows; gama muurolene (100%), beta-caryophyllene (97.60 and 93.14 %), alpha-terpinenyl acetate (91.95 and 83.16 %), geranyl acetate (94.81 and 43.87 %), and terpinen-4-ol (94.40 and 57.00 %). CONCLUSION: The findings of this study indicate the imminent application of simultaneous saccharification and fermentation for the enhancement of bioactivities of terpenoids.
Antiplasmodial potential and GC-MS fingerprint of endophytic fungal extracts derived from Cameroonian Annona muricata.[Pubmed:30738118]
J Ethnopharmacol. 2019 May 10;235:111-121.
ETHNOPHARMACOLOGICAL RELEVANCE: Annona muricata (Annonaceae) is a commonly used medicinal plants in Cameroonian traditional medicines to treat various diseases including malaria. Previous studies have shown that extracts from this plant have antiplasmodial activity. AIM OF THE STUDY: This study aimed to explore the endophyic fungi associated with some parts of this plant for their ability to produce antiplasmodial metabolites. MATERIALS AND METHODS: One hundred and fifty-two endophytic fungi isolated from twelve different organs of A. muricata were cultured and the ethyl acetate extracts of conditioned media screened for antiplasmodial activity using the 96-well microtiter plate format SYBR green florescence assay against Chloroquine-sensitive Pf3D7 and Chloroquine-resistant PfINDO/PfDd2 strains of Plasmodium falciparum. RESULTS: Twenty-seven (17.76%) of fungi tested were found to completely inhibit the growth of Plasmodium parasites at 10microg/mL. The 5.8S rDNA sequencing data revealed the strongly active (IC50 < 2microg/mL against at least 2 P. falciparum strains) isolates to be Trichoderma afroharzianum AMrb7, Penicillium citrinum AMrb11, Neocosmospora rubicola AMb22, Penicillium tropicum AMb3, Penicillium citrinum AMrb23, Aspergillus versicolor AMb7, and Fusarium sp AMst1. Of these, the extracts from Penicillium citrinum AMrb11 (IC50 0.84-0.93mug/mL) and Neocosmospora rubicola AMb22 (IC50 0.39-1.92mug/mL) showed the highest promise against all three plasmodial strains with selectivity indices ranging from 34.71 to 180.97. Dynamic head space GC-MS analysis of ethyl acetate extracts of promising fungi revealed broad-spectrum antimicrobial compounds such as Penicidin, Aromadendrene, Ethyl p-methoxycinnamate, 2-Coumaranone and 2-Methyl resorcinol. CONCLUSION: These results have opened new avenues for discovery of novel antimalarial lead compounds from endophytic fungi associated with Annona muricata - a medicinally important plant.
Phytochemistry of Three Ecuadorian Lamiaceae: Lepechinia heteromorpha (Briq.) Epling, Lepechinia radula (Benth.) Epling and Lepechinia paniculata (Kunth) Epling.[Pubmed:30577466]
Plants (Basel). 2018 Dec 20;8(1). pii: plants8010001.
In this research, the leaves of Lepechinia heteromorpha (Briq.) Epling, Lepechinia radula (Benth.) Epling and Lepechinia paniculata (Kunth) Epling have been collected in order to perform a phytochemical study. The first species was distilled to obtain a novel essential oil (EO), while the others were submitted to ethyl acetate extraction and secondary metabolite isolation. The chemical composition of the EO from L. heteromorpha has been investigated by Gas Chromatography-Mass Spectrometry (GC-MS) and Gas Chromatography with Retention Indices (GC(RI)), identifying 25 constituents. A major compound, (-)-ledol (21.2%), and a minor compound, (-)-caryophyllene oxide (1.0%), were isolated from the EO and their structures confirmed by Nuclear Magnetic Resonance (NMR) spectroscopy. Other major constituents of the EO were viridiflorene (27.3%), (E,E)-alpha-farnesene (1.4%), spirolepechinene and (E)-beta-caryophyllene (7.1% each), allo-Aromadendrene (6.1%), camphor (1.7%), limonene (1.3%) and beta-phellandrene (4.6%). The enantiomeric composition of the EO monoterpene fraction was also studied, determining the enantiomeric excess and distribution of alpha-pinene, limonene, beta-phellandrene and camphor. The ethyl acetate extract of L. radula and L. paniculata were fractionated by column chromatography. Spathulenol, angustanoic acid E and 5-hydroxy-4',7-dimethoxy flavone were isolated from L. radula extract; ledol, guaiol and (-)-carnosol were found in L. paniculata.
Induction of apoptosis by essential oil from P. missionis in skin epidermoid cancer cells.[Pubmed:30466977]
Phytomedicine. 2018 Nov 15;50:184-195.
BACKGROUND: The genus Pamburus (Rutaceae) comprises the only species, Pamburus missionis (Wight) Swingle. Pamburus missionis is traditionally used in the treatment of swellings, chronic rheumatism, paralysis and puerperal diseases. PURPOSE: The present study investigates the cancer chemotherapeutic potential of essential oil (EO) from P. missionis. METHODS: EO was isolated by steam distillation and chemical composition was determined by GC-MS. Cell viability was used to detect cytotoxic activity. Mechanism of cell death was studied using Annexin V-FITC/PI binding, cell cycle analysis, measurement of MMP and ROS generation by flow cytometry. Expression of apoptosis related proteins was investigated by western blot. RESULTS: GC-MS analysis of the essential oil revealed the presence of 51 components. The major components were beta-Caryophyllene, 4(14),11-Eudesmadiene, Aromadendrene oxide-(2) and Phytol. EO inhibited the growth and colony formation ability of A431 and HaCaT cells. EO treatment induced nuclear condensation and loss of membrane integrity, DNA fragmentation, increase in sub-G1 DNA content and increase in intracellular ROS level. Inhibition of intracellular ROS by ascorbic acid and N-acetyl cysteine treatment blocked EO induced apoptosis, revealing that apoptotic activity was by ROS accumulation. EO induced apoptosis was found to be due to the loss of mitochondrial membrane potential (DeltaPsim), increase in Bax/Bcl-2 ratio, release of cytochrome c and activation of caspases (cleaved form of caspase-3, caspase-8, caspase-9) and by PARP cleavage. CONCLUSION: The present study revealed cancer chemotherapeutic potential of EO from P. missionis. EO induces cell death through intrinsic (mitochondrial) and extrinsic apoptotic pathway in A431 and HaCaT cells. These results suggest that EO could be used as a potential therapeutic agent for the treatment of skin epidermoid cancer.
Antibacterial and antiproliferative activities of the fresh leaf essential oil of Psidium guajava L. (Myrtaceae).[Pubmed:30462815]
Braz J Biol. 2018 Nov 14. pii: S1519-69842018005031104.
This study evaluated the antibacterial and antiproliferative activities of the essential oil of Psidium guajava leaves (PG-EO), traditionally used in folk medicine. The essential oil was obtained from fresh leaves by hydrodistillation, using a modified Clevenger apparatus. The major PG-EO chemical constituents were identified by GC-MS and GC-FID as being beta-caryophyllene (16.1%), alpha-humulene (11.9%), Aromadendrene oxide (14.7%), delta-selinene (13.6%), and selin-11-en-4alpha-ol (12.5%). The antibacterial activity of the essential oil of P. guajava leaves was determined in terms of its minimum inhibitory concentrations (MIC) using the broth microdilution method in 96-well microplates. PG-EO had moderate activity against Streptococcus mutans (MIC = 200 microg/mL), S. mitis (MIC = 200 microg/mL), S. sanguinis (MIC = 400 microg/mL), S. sobrinus (MIC = 100 microg/mL), and S. salivarius (MIC = 200 microg/mL). The antiproliferative activity was evaluated against different tumor cell lines: breast adenocarcinoma (MCF-7), human cervical adenocarcinoma (HeLa), and human gliobastoma (M059J). A normal human cell line (GM07492A, lung fibroblasts) was included. The antiproliferative activity was evaluated using the XTT assay and the results were expressed as IC50. The essential oil showed significantly lower IC50 values against MCF-7 and M059J lines than that obtained for the normal line, showing selectivity. Our results suggest that the essential oil of Psidium guajava L. has promising biological activities and can be considered a new source of bioactive compounds.
Understorey Rhododendron tomentosum and Leaf Trichome Density Affect Mountain Birch VOC Emissions in the Subarctic.[Pubmed:30185795]
Sci Rep. 2018 Sep 5;8(1):13261.
Subarctic vegetation is composed of mountain birch [Betula pubescens ssp. czerepanovii (MB)] forests with shrubs and other species growing in the understorey. The effects of the presence and density of one understorey shrub, Rhododendron tomentosum (RT), on the volatile emissions of MB, were investigated in a Finnish subarctic forest site in early and late growing season. Only MB trees with an RT-understorey emitted the RT-specific sesquiterpenoids, palustrol, ledol and Aromadendrene. Myrcene, which is the most abundant RT-monoterpene was also emitted in higher quantities by MB trees with an RT-understorey. The effect of RT understorey density on the recovery of RT compounds from MB branches was evident only during the late season when sampling temperature, as well as RT emissions, were higher. MB sesquiterpene and total emission rates decreased from early season to late season, while monoterpene emission rate increased. Both RT and MB terpenoid emission rates were linked to density of foliar glandular trichomes, which deteriorated over the season on MB leaves and emerged with new leaves in the late season in RT. We show that sesquiterpene and monoterpene compounds emitted by understorey vegetation are adsorbed and re-released by MB, strongly affecting the MB volatile emission profile.
Synergistic interaction of beta-caryophyllene with aromadendrene oxide 2 and phytol induces apoptosis on skin epidermoid cancer cells.[Pubmed:30166097]
Phytomedicine. 2018 Aug 1;47:121-134.
BACKGROUND: Pamburus missionis (Wight) Swingle (Rutaceae) is traditionally used in the treatment of swellings, chronic rheumatism, paralysis and puerperal diseases. In a previous study the authors demonstrated apoptotic activity of Pamburus missionis essential oil (EO) on A431 and HaCaT cells. The major components of EO were beta-caryophyllene (25.40%), 4(14),11- eudesmadiene (7.17%), Aromadendrene oxide 2 (14.01%) (AO-(2) and phytol (6.88%). PURPOSE OF STUDY: To investigate the role as well as the interactions among EO components inducing apoptosis in A431 and HaCaT cells. METHODS: Isobolographic analysis and combination index methods were used to detect the type of interactions among the essential oil (EO) components. Cell viability was used to detect cytotoxic activity. Mechanism of cell death was studied using Annexin V-FITC/PI binding assay, cell cycle analysis, measurement of MMP and ROS generation by flow cytometry. Expression of apoptosis associated proteins was investigated by western blot. RESULTS: Combination of P. missionis EO components: beta-caryophyllene/ Aromadendrene oxide 2 (beta-C/AO-(2)), beta-caryophyllene/phytol (beta-C/P) and Aromadendrene oxide 2 /phytol (AO-(2)/P) inhibited growth and colony formation ability of skin epidermoid A431 and precancerous HaCaT cells. Synergistic interaction was observed between beta-C/AO-(2) and beta-C/P combination while AO-(2)/P exhibited an additive effect. Combination of components induced chromatin condensation, phosphatidylserine externalisation, increase in sub-G1 DNA content, cell cycle arrest at G0/G1 phase and intracellular ROS accumulation. Inhibition of intracellular ROS by N-acetyl cysteine treatment blocked apoptosis induced by the combinations. The combinations induced apoptosis in A431 and HaCaT cells mediated by loss of mitochondrial membrane potential (DeltaPsim), increase in Bax/Bcl-2 ratio, release of cytosolic cytochrome c and activation of caspases (cleaved form of caspase-3, caspase-8, caspase-9) and by PARP cleavage. CONCLUSION: The present study demonstrates interactions among beta-C, AO-(2) and P in the induction of apoptosis on A431 and HaCaT cells. These data suggest the combination of beta-caryophyllene with Aromadendrene oxide 2 and phytol could be potential therapeutics for the treatment of skin epidermoid cancer and precancerous cells.
Gas/Particle Partitioning Constants of Nicotine, Selected Toxicants, and Flavor Chemicals in Solutions of 50/50 Propylene Glycol/Glycerol As Used in Electronic Cigarettes.[Pubmed:30113826]
Chem Res Toxicol. 2018 Sep 17;31(9):985-990.
For an electronic cigarette (e-cigarette) aerosol with known total particulate matter concentration (TPM, mug/m(3)), predictions of the fractions of some compound i in the gas and particle phases ( fg, i and fp, i) at equilibrium can be made based on Kp, i (m(3)/mug), the compound-dependent gas/particle partitioning equilibrium constant. fg, i and fp, i affect the modes and locations of deposition in the respiratory tract. Kp, i depends inversely on (1) the pure compound liquid vapor pressure ( pL, i(o)), (2) mole fraction activity coefficient (zeta i) in the absorbing liquid, and (3) mean molecular weight of the absorbing liquid (MW). Kp, i values were measured at 20 degrees C for 32 compounds as spiked into simulated e-cigarette liquids prepared as 50/50 mixtures (by weight) of propylene glycol (PG) and glycerol (GL). Kp, i values at 37 degrees C were estimated. The 32 compounds were nicotine (in free-base form), seven toxicants (propanal, acetone, hydroxyacetone, benzene, toluene, p-xylene, and ethylbenzene), and 24 flavor chemicals (2,3-pentanedione ("acetyl propionyl"), isobutyl acetate, ethyl butyrate, butyl butyrate, isoamyl acetate, 2,3-dimethylpyrazine, 3-methyl-1-butanol, limonene, 2,3,5-trimethylpyrazine, p-cymene, benzaldehyde, ( Z)-3-hexen-1-ol, menthol, 2-acetylpyrrole, benzyl alcohol, methyl salicylate, cinnamaldehyde, methyl anthranilate, (+)-Aromadendrene, cinnamyl alcohol, methyl cinnamate, maltol, ethyl maltol, and coumarin). The measured log Kp, i values were found to be generally correlated with literature values of log pL, i(o); the scatter is caused by variation in zeta i between approximately 1 and approximately 1000. Kp measurements were attempted, but values were not reported for acetaldehyde, 2,3-butanedione (diacetyl), vanillin, and ethyl vanillin. Acetaldehyde was found to form significant amounts of its cyclic trimer and cyclic tetramer; for diacetyl, the evidence suggested significant amounts of reaction products, possibly hemiketals and ketals with PG/GL, and for vanillin and ethyl vanillin, the Kp values are large and accordingly more difficult to measure. fg values are calculated using a range of Kp and TPM values.
Benzaldehyde Synergizes the Response of Female Xyleborinus saxesenii (Coleoptera: Curculionidae, Scolytinae) to Ethanol.[Pubmed:29767753]
J Econ Entomol. 2018 May 15. pii: 4996095.
The ambrosia beetle, Xyleborinus saxesenii Ratzeburg (Coleoptera: Curculionidae, Scolytinae), infests physiologically stressed apple and peach trees in Korea. Dispersing females utilize the degradation product ethanol and host-related volatiles to locate and colonize new host trees. We examined the extent to which 12 chemicals emitted from fruit trees act synergistically with ethanol to attract X. saxesenii. The addition of benzaldehyde to ethanol significantly increased beetle attraction, although benzaldehyde was not attractive by itself. The addition of (-)-alpha-pinene, ethyl butyrate, ethyl isovalerate, (R)-(+)-limonene, 3-methyl-1-butanol, ethyl tiglate, (+)-Aromadendrene, vanillin, 2-butanol, styrene, or ethyl 3,3-dimethylacrylate to ethanol had no effect on beetle attraction. In a dose-response test, the addition of 5-50% benzaldehyde doses synergistically increased the number of beetle captures; however, trap catches did not increase as the benzaldehyde dosage increased. The synergistic influence of benzaldehyde on beetle response to ethanol was lower in early spring than in late summer to early fall, probably because synthetic benzaldehyde emissions from field lures were overwhelmed by background levels of natural benzaldehyde emitted from peach twigs in the flowering stage.
Chemical characterization and bioherbicidal potential of the essential oil from the leaves of Unonopsis guatterioides (A.DC.) R.E.Fr. (Annonaceae).[Pubmed:29741113]
Nat Prod Res. 2018 May 9:1-5.
The chemical composition and the phytotoxicity potential of the essential oil from leaves of Unonopsis guatterioides (A.DC.) R.E.Fr. (Annonaceae) was investigated. Gas chromatography/mass spectrometry analyses revealed 16 constituents representing 99.50% of the total essential oil, composed mainly of sesquiterpenes. alpha-copaene, bicyclogermacrene and trans-caryophyllene were the major components (15.7% each), followed by alpha-humullene, allo-Aromadendrene and (+)-spathulenol (9.0, 8.4 and 7.3%, respectively). The essential oil inhibited seed germination and growth in both monocotyledon (Allium cepa) and dicotyledon (Lactuca sativa) models, pointing to a promising application of this oil obtained from the leaves of U. guatterioides as a new bioherbicide.
Aromadendrene oxide 2, induces apoptosis in skin epidermoid cancer cells through ROS mediated mitochondrial pathway.[Pubmed:29407546]
Life Sci. 2018 Mar 15;197:19-29.
AIMS: Aromadendrene oxide 2 (AO-(2)) is an oxygenated sesquiterpene naturally found as a chemical component of essential oils. In the present study anticancer activity of AO-(2) has been investigated on A431 human epidermoid cancer and precancerous HaCaT cells. MATERIAL AND METHODS: Cell viability was used to detect cytotoxic activity. Mechanism of cell death induced by AO-(2) treatments was studied using Annexin V-FITC/PI binding, cell cycle analysis, measurement of MMP and ROS generation by flow cytometry. Expression of apoptosis related proteins was investigated by western blot. KEY FINDINGS: AO-(2) inhibited the growth and colony formation ability of A431 and HaCaT cells in concentration dependent manner. It induced cell cycle arrest at G0/G1 phase and apoptosis through intracellular ROS accumulation. Inhibition of intracellular ROS by ascorbic acid and N-acetyl cysteine treatment completely blocked apoptotic effect. N-acetyl cysteine treatment significantly reversed G0/G1 arrest induced by AO-(2). AO-(2) treatment caused loss of mitochondrial membrane potential (DeltaPsim), increase in Bax/Bcl-2 ratios, cytochrome c release, activation of caspases (cleaved caspase-3 and caspase-9) and PARP cleavage. AO-(2) also significantly inhibited the growth of multicellular tumor spheroids of A431 and HaCaT cells. SIGNIFICANCE: The results of the present study reveals that AO-(2) a chemical component of essential oils induces apoptosis in A431 and HaCaT cells.
Molecular basis of peripheral olfactory sensing during oviposition in the behavior of the parasitic wasp Anastatus japonicus.[Pubmed:28912112]
Insect Biochem Mol Biol. 2017 Oct;89:58-70.
Anastatus japonicus is a parasitic wasp and natural enemy of the litchi pest Tessaratoma papillosa, and for decades in China, A. japonicus has been mass-reared inside the eggs of Antheraea pernyi to control T. papillosa. A series of experiments was performed to explore the olfactory mechanism underlying the oviposition behavior of A. japonicus. First, a transcriptomic analysis was performed on the antennae of A. japonicus, and the resulting assemblies led to the generation of 70,473 unigenes. Subsequently, 21,368 unigenes were matched to known proteins, 48 odorant receptors (ORs) (including Orco) and 13 antennal ionotropic receptors (IRs) (including the co-receptors IR8a and IR25a) were identified and predicted to form complete open reading frames (ORFs). The FPKM (fragments per Kb per million reads) values and RT-PCR results showed that AjapOrco, AjapOR10, AjapOR27, AjapOR33 and AjapOR35 were either highly abundant or expressed specifically in the olfactory organs. Furthermore, AjapOrco silencing resulted in a significant decrease in both the parasitism rate and the host-seeking time of A. japonicus, whereas dsRNA injection showed that IR8a and IR25a did not produce significant behavioral changes, suggesting that the oviposition behavior of A. japonicus is more reliant on OR-based pathways than IR-based pathways. Our previous GC-MS data derived twenty-nine compounds which were abundent from these host plants and host insects. We performed electrophysiological and oviposition assays on A. japonicus, and eight odorants were found to elicit a significant electroantennogram (EAG) response. Among these odorants, beta-Caryophyllene, Undecane, (E)-alpha-Farnesene (+)-Aromadendrene and Cis-3-Hexen-ol had strong attractant effects on oviposition, whereas 2-Ethyl-1-Hexan-ol, Ethyl Acetate and alpha-Caryophyllene had a strong repellant effects. Thus, these chemicals might influence oviposition guidance/repulsion behavior in A. japonicus. To further explore the target ORs that are tuned to the functional odorants, the nine candidate ORs described above were silenced by RNA interference, and the results showed that a large decrease in the EAG response of all the tested functional odorants in the AjapOrco-silencing group. In addition, the AjapOR35-silencing group showed a significant decrease in the EAG response to beta-Caryophyllene and (E)-alpha-Farnesene, indicating that AjapOR35 is tuned to these two oviposition attractants beta-Caryophyllene and (E)-alpha-Farnesene. Further binary-choice oviposition assays showed that the oviposition attractant effect of beta-Caryophyllene and (E)-alpha-Farnesene vanished after AjapOR35 was silenced, indicating that the emission of these attractants from host plants can guide A. japonicus to locate eggs for ovipositioning and indicated that AjapOR35 is correlated with the olfactory detection oviposition behavior of this species. This study provides a better understanding of the molecular basis and functional chemicals underlying the oviposition behavior of A. japonicus, and the results may help improve biocontrol approaches.
Comparative toxicity of essential oil and blends of selected terpenes of Ocotea species from Pernambuco, Brazil, against Tetranychus urticae Koch.[Pubmed:28767894]
An Acad Bras Cienc. 2017 Jul-Sep;89(3):1417-1429.
Essential oils from the leaves of two species of the genus Ocotea that occur in the Atlantic Forest in the state of Pernambuco, Brazil, were analyzed using gas chromatography-mass spectrometry. The acaricidal activity of these oils as well as 11 selected components and blends were evaluated in fumigation and residual contact tests against the two-spotted spider mite (Tetranychus urticae). Sixty-seven constituents were identified, totaling 97.3 +/- 0.3% and 97.8 +/- 0.5% of the oils from O. duckei and O. glomerata, respectively. Sesquiterpene was the dominant class. The compounds beta-caryophyllene (18.6 +/- 0.1%) and Aromadendrene (17.3 +/- 0.6%) were the main constituents of the oils from O. duckei and O. glomerata, respectively. Acaricidal action varied depending on the method employed, species and chemical nature of the selected constituents. The mites were susceptible to the oils and chemical constituents using the fumigation method. The O. duckei oil was respectively 2.5-fold and 1.5-fold more toxic than the O. glomerata oil using the fumigation and residual contact methods. Among the selected constituents, beta-caryophyllene was the most toxic, independently of the method employed. The individual toxicity of the selected compounds and their blends as well as the role of these constituents in the overall toxicity of the essential oils are also discussed.
Cytotoxic Essential Oils from Eryngium campestre and Eryngium amethystinum (Apiaceae) Growing in Central Italy.[Pubmed:28332760]
Chem Biodivers. 2017 Jul;14(7).
Eryngium campestre and E. amethystinum are thorny herbs belonging to the Apiaceae family and spontaneously growing in stony pastures and dry meadows, preferentially on calcareous substrates. In the Mediterranean countries, these plants have been used as a food or traditional remedies to treat various ailments. In the present work, we have analyzed the chemical composition of the essential oils distilled from the aerial parts by GC-FID and GC/MS, and evaluated their cytotoxic effects on a panel of human cancer cells, namely A375 (human malignant melanoma), MDA-MB 231 cells (human breast adenocarcinoma), and HCT116 cells (human colon carcinoma), by the MTT assay. Furthermore, the Eryngium essential oils were evaluated for antioxidant and acetylcholinesterase (AChE) activities. The two essential oils were rich in sesquiterpene hydrocarbons, with germacrene D as the major compound, accompanied by allo-Aromadendrene, beta-elemene, spathulenol, and ledol. They turned out to be highly cytotoxic on the tumor cells, with IC50 values (1.65 - 5.32 and 1.57 - 2.99 mug/ml for E. amethystinum and E. campestre, respectively) comparable or close to those of the anticancer drug cisplatin. The E. amethystinum essential oil exhibited a moderate antioxidant activity, whereas that of E. campestre a weak AChE inhibition.


