Apigenin triacetateCAS# 3316-46-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3316-46-9 | SDF | Download SDF |
| PubChem ID | 18721.0 | Appearance | Powder |
| Formula | C21H16O8 | M.Wt | 396.35 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
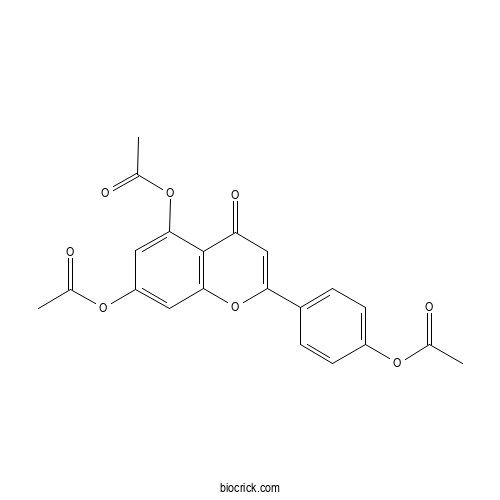
| Chemical Name | [4-(5,7-diacetyloxy-4-oxochromen-2-yl)phenyl] acetate | ||
| SMILES | CC(=O)OC1=CC=C(C=C1)C2=CC(=O)C3=C(O2)C=C(C=C3OC(=O)C)OC(=O)C | ||
| Standard InChIKey | IVXFOQQPPONQTB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H16O8/c1-11(22)26-15-6-4-14(5-7-15)18-10-17(25)21-19(28-13(3)24)8-16(27-12(2)23)9-20(21)29-18/h4-10H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Apigenin triacetate Dilution Calculator

Apigenin triacetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.523 mL | 12.6151 mL | 25.2302 mL | 50.4605 mL | 63.0756 mL |
| 5 mM | 0.5046 mL | 2.523 mL | 5.046 mL | 10.0921 mL | 12.6151 mL |
| 10 mM | 0.2523 mL | 1.2615 mL | 2.523 mL | 5.046 mL | 6.3076 mL |
| 50 mM | 0.0505 mL | 0.2523 mL | 0.5046 mL | 1.0092 mL | 1.2615 mL |
| 100 mM | 0.0252 mL | 0.1262 mL | 0.2523 mL | 0.5046 mL | 0.6308 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Homodihydrocapsaicin II
Catalog No.:BCX0998
CAS No.:71239-21-9
- Neopuerarin B
Catalog No.:BCX0997
CAS No.:1150314-39-8
- Hericene D
Catalog No.:BCX0996
CAS No.:1343477-87-1
- Maltononaose
Catalog No.:BCX0995
CAS No.:6471-60-9
- Maltodecaose
Catalog No.:BCX0994
CAS No.:6082-21-9
- 1,3-Disinapoylglucose
Catalog No.:BCX0993
CAS No.:1423128-15-7
- S-Isogambogic acid
Catalog No.:BCX0992
CAS No.:942623-57-6
- Forbesione
Catalog No.:BCX0991
CAS No.:180961-63-1
- (9Z,11E)-13-Oxo-9,11-octadecadienoic Acid
Catalog No.:BCX0990
CAS No.:54739-30-9
- 5-Methoxyisosakuranin
Catalog No.:BCX0989
CAS No.:59942-61-9
- Violaxanthin
Catalog No.:BCX0988
CAS No.:126-29-4
- Antheraxanthin
Catalog No.:BCX0987
CAS No.:640-03-9
- Anisomelic acid
Catalog No.:BCX1000
CAS No.:59632-76-7
- Ovatodiolide
Catalog No.:BCX1001
CAS No.:3484-37-5
- Neohesperidose
Catalog No.:BCX1002
CAS No.:17074-02-1
- α-Glucosyl Hesperidin
Catalog No.:BCX1003
CAS No.:161713-86-6
- Hericene B
Catalog No.:BCX1004
CAS No.:157207-55-1
- Hericene A
Catalog No.:BCX1005
CAS No.:157207-54-0
- Euphorbetin
Catalog No.:BCX1006
CAS No.:35897-99-5
- Alitame hydrate
Catalog No.:BCX1007
CAS No.:99016-42-9
- Isomaltooctaose
Catalog No.:BCX1008
CAS No.:35867-37-9
- Isomaltoheptaose
Catalog No.:BCX1009
CAS No.:6513-12-8
- Isomaltohexaose
Catalog No.:BCX1010
CAS No.:6175-02-6
- Isomaltopentaose
Catalog No.:BCX1011
CAS No.:6082-32-2
Neuroprotective Efficacy of Phytoconstituents of Methanolic Shoots Extract of Calligonum polygonoides L. in Hypercholesterolemia-associated Neurodegenerations.[Pubmed:38571361]
Endocr Metab Immune Disord Drug Targets. 2024 Apr 2.
BACKGROUND: Small molecule phytocompounds can potentially ameliorate degenerative changes in cerebral tissues. Thus, the current study aimed to evaluate the neuroprotective efficacy of phytocompounds of methanolic shoots extract of Calligonum polygonoides L. (MSECP) in hypercholesterolemia-associated neurodegenerations. METHODS: Phytochemical screening of the extract was made by LCMS/MS and validated by a repository of the chemical library. The hypercholesterolemia was induced through the intraperitoneal administration of poloxamer-407 with a high-fat diet. The in-silico assessments were accomplished by following the molecular docking, ADME and molecular dynamics. MMPBSA and PCA (Principal Component Analysis) analyzed the molecular dynamics simulations. Consequently, in-vivo studies were examined by lipid metabolism, free radical scavenging capabilities and histopathology of brain tissues (cortex and hippocampus). RESULTS: 22 leading phytocompounds were exhibited in the test extract, as revealed by LCMS/ MS scrutiny. Molecular docking evaluated significant interactions of Apigenin triacetate with target proteins (HMGCR (HMG-CoA reductase), (AChE-Acetylcholinesterase) and (BuChE- Butyrylcholinesterase). Molecular dynamics examined the interactions through assessments of the radius of gyration, RSMD, RSMF and SASA at 100 ns, which were further analyzed by MMPBSA (Molecular Mechanics Poisson-Boltzmann) and PCA (Principal Component Analysis). Accordingly, the treatment of test extract caused significant alterations in lipid profile, dyslipidemia indices, antioxidant levels and histopathology of brain tissues. CONCLUSION: It can be concluded that Apigenin triacetate is a potent phytoconstituent of MSEPC and can interact with HMGCR, AChE, and BuChE, which resulted in improved hypercholesterolemia along with neuroprotective ameliorations in the cortex and hippocampus.
Mutagenicities of 61 flavonoids and 11 related compounds.[Pubmed:7021146]
Environ Mutagen. 1981;3(4):401-19.
The mutagenicities of 61 flavonoids (naturally occurring flavonoid aglycones and flavonal glycosides and synthetic flavonoids) and those of 11 compounds structurally related to flavonoids were tested with Salmonella typhimurium strains TA100 and TA98. Among the 22 flavone derivatives tested, only wogonin was strongly mutagenic, while five derivatives, Apigenin triacetate, acacetin, chrysoeriol, pedalitin, and pedalitin tetraacetate, were only weakly mutagenic. Two bisflavonyl derivatives, neither of which has a 3-hydroxyl group, were not mutagenic. Of the 16 flavonol derivatives tested, all except 3-hydroxyflavone and the tetra- and penta-methyl ethers of quercetin were mutagenic. Of the five flavanone derivatives tested, only 7,4-dihydroxyflavanone was mutagenic, showing weak activity. Of the four flavanolol derivatives tested, hydrorobinetin and taxifolin were weakly mutagenic. Of the six isoflavone derivatives tested, tectorigenin was weakly mutagenic. Of the 11 compounds in the miscellaneous group structurally related to flavonoids, only isoliquiritigenin was mutagenic, showing weak activity. For the emergence of strong mutagenicity, the double bond between positions 2 and 3 and the hydroxyl group at position 3 are required, except in wogonin, which does not have a hydroxyl group at position 3 but is strongly mutagenic to TA100. The 3-O-acetyl ester of flavonol, quercetin, was mutagenic with S9 mix, but 3-O-methyl ethers were not. Six flavonol glycosides, three quercetin glycosides and three kaempferol glycosides were mutagenic after preincubation with "hesperidinase," a crude extract of Aspergillus niger. Of 66 flavonoid agylcones and compounds structurally related to flavonoids, quercetin was the strongest mutagen. The carcinogenicity of this compound should be clarified because it is ubiquitously found in vegetables.


