Ansamitocin P-3CAS# 66547-09-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 66547-09-9 | SDF | Download SDF |
| PubChem ID | 6446789 | Appearance | White crystalline powder |
| Formula | C32H43ClN2O9 | M.Wt | 635.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (157.45 mM; Need ultrasonic) | ||
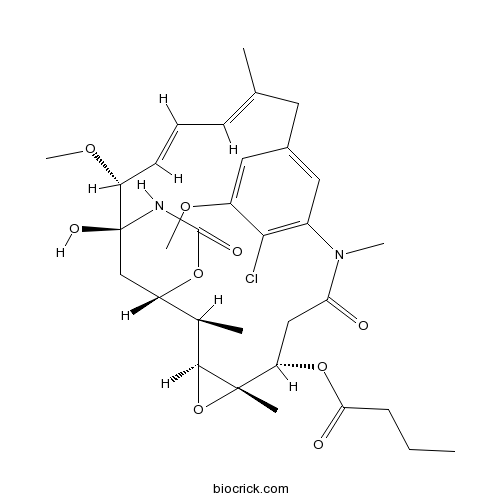
| Chemical Name | [(1S,2R,3S,5S,6S,16E,18E,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaen-6-yl] butanoate | ||
| SMILES | CCCC(=O)OC1CC(=O)N(C2=C(C(=CC(=C2)CC(=CC=CC(C3(CC(C(C4C1(O4)C)C)OC(=O)N3)O)OC)C)OC)Cl)C | ||
| Standard InChIKey | WLKHTIAFMSHJLG-BYXOJEECSA-N | ||
| Standard InChI | InChI=1S/C32H43ClN2O9/c1-8-10-27(37)43-25-16-26(36)35(5)21-14-20(15-22(40-6)28(21)33)13-18(2)11-9-12-24(41-7)32(39)17-23(42-30(38)34-32)19(3)29-31(25,4)44-29/h9,11-12,14-15,19,23-25,29,39H,8,10,13,16-17H2,1-7H3,(H,34,38)/b12-9+,18-11+/t19-,23+,24-,25+,29+,31+,32+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ansamitocin P-3 Dilution Calculator

Ansamitocin P-3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5745 mL | 7.8723 mL | 15.7446 mL | 31.4891 mL | 39.3614 mL |
| 5 mM | 0.3149 mL | 1.5745 mL | 3.1489 mL | 6.2978 mL | 7.8723 mL |
| 10 mM | 0.1574 mL | 0.7872 mL | 1.5745 mL | 3.1489 mL | 3.9361 mL |
| 50 mM | 0.0315 mL | 0.1574 mL | 0.3149 mL | 0.6298 mL | 0.7872 mL |
| 100 mM | 0.0157 mL | 0.0787 mL | 0.1574 mL | 0.3149 mL | 0.3936 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Propacetamol hydrochloride
Catalog No.:BCC9129
CAS No.:66532-86-3
- Benzoin isopropyl ether
Catalog No.:BCC8856
CAS No.:6652-28-4
- MSOP
Catalog No.:BCC6801
CAS No.:66515-29-5
- Bicifadine hydrochloride
Catalog No.:BCC7925
CAS No.:66504-75-4
- Amantadine HCl
Catalog No.:BCC4465
CAS No.:665-66-7
- JW 55
Catalog No.:BCC2453
CAS No.:664993-53-7
- H-D-Lys(Boc)-OMe.HCl
Catalog No.:BCC2990
CAS No.:66494-53-9
- Kaempferol 3-O-(6''-O-acetyl)glucoside-7-O-rhamnoside
Catalog No.:BCN1385
CAS No.:66465-24-5
- 2-(2,4-Diaminophenoxy)ethanol dihydrochloride
Catalog No.:BCN8497
CAS No.:66422-95-5
- 6-Amino-1,3-dimethyluracil
Catalog No.:BCC8755
CAS No.:6642-31-5
- 13-Hydroxyoxyberberine
Catalog No.:BCN3355
CAS No.:66408-27-3
- Pamoic acid disodium salt
Catalog No.:BCC7909
CAS No.:6640-22-8
- Augustifolin
Catalog No.:BCN3232
CAS No.:66548-01-4
- CL 218872
Catalog No.:BCC7162
CAS No.:66548-69-4
- ent-3beta-Hydroxykaur-16-en-19-oic acid
Catalog No.:BCN6472
CAS No.:66556-91-0
- Tsugafolin
Catalog No.:BCN4026
CAS No.:66568-97-6
- Forskolin
Catalog No.:BCN2332
CAS No.:66575-29-9
- Dihydrorotenone
Catalog No.:BCN2726
CAS No.:6659-45-6
- Agaric acid
Catalog No.:BCC9216
CAS No.:666-99-9
- Boc-Phenylalaninol
Catalog No.:BCC2718
CAS No.:66605-57-0
- RU 24969
Catalog No.:BCC5423
CAS No.:66611-26-5
- Risperidone hydrochloride
Catalog No.:BCC4205
CAS No.:666179-74-4
- Risperidone mesylate
Catalog No.:BCC4206
CAS No.:666179-96-0
- 2',4'-Dihydroxy-3,7':4,8'-diepoxylign-7-ene
Catalog No.:BCN6645
CAS No.:666250-52-8
Proteomic studies on anti-tumor agent ansamitocin P-3 producer Actinosynnema pretiosum in response to ammonium and isobutanol.[Pubmed:28382459]
Bioprocess Biosyst Eng. 2017 Jul;40(7):1133-1139.
Our previous work showed that the biosynthesis of Ansamitocin P-3 (AP-3), an anti-tumor agent, by Actinosynnema pretiosum was depressed by ammonium but enhanced by isobutanol in the medium. Here we show proteomics analyses on A. pretiosum in different fermentation conditions with and without ammonium or isobutanol using two-dimensional electrophoresis (2-DE), matrix-assisted laser desorption/ionization, and linear ion trap quadrupole mass spectrometry. Pairwise comparison of repetitive 2-DE maps was performed to find differentially expressed spots, and eight proteins were identified as functionally annotated ones. Among these proteins, D-3-phosphoglycerate dehydrogenase (PGDH) and glyceraldehyde 3-phosphate dehydrogenase showed statistically significant up-regulation in ammonium vs. basic or isobutanol medium, while fatty acid synthetase, histidine-tRNA ligase, transposase, molecular chaperone GroEL, SAM-dependent methyltransferase, and Crp/Fnr family transcriptional regulator were overexpressed in ammonium vs. basic medium. Based on the 2-DE data, exogenous L-serine which could inhibit the PGDH activity was added to the cultures with isobutanol, and a lower AP-3 production was confirmed under 2.5 mM serine addition (24 or 48 h).
Identification and Engineering of Post-PKS Modification Bottlenecks for Ansamitocin P-3 Titer Improvement in Actinosynnema pretiosum subsp. pretiosum ATCC 31280.[Pubmed:28881098]
Biotechnol J. 2017 Nov;12(11).
The type-I polyketide Ansamitocin P-3 (AP-3) is a potent antitumor agent. Its production is most likely hampered by the required multiple substrate supplies and complicated post-PKS modifications in Actinosynnema pretiosum subsp. pretiosum ATCC 31280. For titer improvement, gene ansa30, encoding for a glycosyltransferase competing for the N-demethyl-AP-3 (PND-3) intermediate for AP-3 biosynthesis, was initially inactivated. In the mutant NXJ-22, the AP-3 titer was increased by 66% along with an obvious accumulation of PND-3, indicating that the N-methylation is a rate-limiting step. Alternatively, when abundant upstream intermediate 19-chloroproansamitocin was fed into a PKS mutant, 3-O-acylation was further identified along with the N-methylation as the rate-limiting steps. Subsequent overexpression of N-methyltransferase gene asm10 in NXJ-22 resulted in a 93% increase of AP-3 and a corresponding 92% decrease of PND-3. Additional supplementation of L-methionine, the precursor for SAM biosynthesis, substantially decreased the accumulation of PND-3. In parallel, the 3-O-acylation bottleneck was relieved by feeding with L-valine to NXJ-22, resulting in a 126% increase of AP-3. Eventually, a combined asm10 overexpression and supplementation of L-methionine and L-valine resulted in a 5-fold increase of AP-3, from 42 +/- 2 mg L(-1) to 246 +/- 6 mg L(-1) , without any noticeable accumulation of PND-3.
Effects of modulation of pentose-phosphate pathway on biosynthesis of ansamitocins in Actinosynnema pretiosum.[Pubmed:27173582]
J Biotechnol. 2016 Jul 20;230:3-10.
Ansamitocins, produced by Actinosynnema pretiosum, are a group of maytansinoid antibiotics that block the assembly of tubulin into functional microtubules. The precursors of ansamitocin biosynthesis are generally derived from the Embden-Meyerhof-Parnas (EMP) pathway and the tricarboxylic acid cycle. In this study, central carbon flux distributions were analyzed by (13)C-based flux analysis to reveal the contribution of individual central carbon metabolism pathways. To direct more carbon flux into ansamitocin biosynthesis, pentose phosphate (PP) pathway only and the combination of PP pathway and Entner-Doudoroff (ED) pathway were weakened, respectively. Ansamitocin P-3 (AP-3) productions by both kinds of pathways weakened mutant strains were significantly enhanced in chemically defined medium. In order to draw metabolic flux to the biosynthesis of ansamitocins more efficiently, heterologous phosphoglucomutase was subsequently overexpressed based on a mutant strain with combinational regulation of PP pathway and ED pathway. More fluxes were successfully directed into the UDP-glucose synthetic pathway and the AP-3 production was further improved in this case, reaching approximately 185mg/L in fermentation medium. It was demonstrated that eliminating the bypass pathways and favoring the precursor synthetic pathway could effectively improve ansamitocin production by A. pretiosum, suggesting a promising role of metabolic strategy in improving secondary metabolite production.
Effects of the Methylmalonyl-CoA Metabolic Pathway on Ansamitocin Production in Actinosynnema pretiosum.[Pubmed:27787765]
Appl Biochem Biotechnol. 2017 Mar;181(3):1167-1178.
Ansamitocins, which may have antitumor activity, are important secondary metabolites produced by Actinosynnema pretiosum sp. auranticum ATCC 31565. As one of the precursors for ansamitocin biosynthesis, methylmalonyl-CoA may be a critical metabolic node for secondary metabolism in A. pretiosum. In this study, we investigated two key enzymes related to the methylmalonyl-CoA metabolic pathway: methylmalonyl-CoA mutase (MCM) and propionyl-CoA carboxylase (PCC). For MCM, inactivation of the asm2277 gene (encoding the large subunit of MCM) resulted in 3-fold increase in Ansamitocin P-3 (AP-3) production (reaching 70 mg/L) compared with that in wild-type A. pretiosum. The three genes responsible for PCC were asm6390, encoding propionyl-CoA carboxylase beta chain, and asm6229 and asm6396, which encoded biotin carboxylases, respectively. Heterogeneous overexpression of the amir6390 gene alone and concurrent overexpression of amir6390 with both amir6396 and amir6229 were carried out, and the resulting engineered strains could produce AP-3 at levels that were 1.6-fold and 3-fold (28.3 and 51.5 mg/L in flask culture, respectively) higher than that in the wild-type strain. These results suggested that eliminating the bypass pathways and favoring the precursor synthetic pathway could effectively increase ansamitocin production in A. pretiosum.


