AnnonacinCAS# 111035-65-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111035-65-5 | SDF | Download SDF |
| PubChem ID | 354398 | Appearance | Powder |
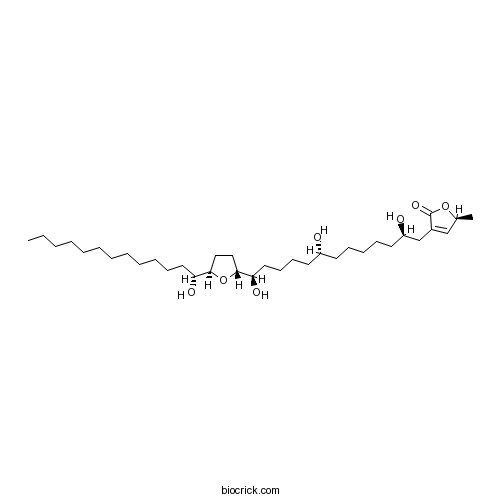
| Formula | C35H64O7 | M.Wt | 596.89 |
| Type of Compound | Lipids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-methyl-4-[(2R,8R,13R)-2,8,13-trihydroxy-13-[(2R,5R)-5-[(1R)-1-hydroxytridecyl]oxolan-2-yl]tridecyl]-2H-furan-5-one | ||
| SMILES | CCCCCCCCCCCCC(C1CCC(O1)C(CCCCC(CCCCCC(CC2=CC(OC2=O)C)O)O)O)O | ||
| Standard InChIKey | XNODZYPOIPVPRF-CGWDHHCXSA-N | ||
| Standard InChI | InChI=1S/C35H64O7/c1-3-4-5-6-7-8-9-10-11-15-21-31(38)33-23-24-34(42-33)32(39)22-17-16-19-29(36)18-13-12-14-20-30(37)26-28-25-27(2)41-35(28)40/h25,27,29-34,36-39H,3-24,26H2,1-2H3/t27-,29+,30+,31+,32+,33+,34+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Annonacin is mitochondrial complex I inhibitor, reported to be more toxic than rotenone to mesencephalic neurons; can cause significant cell death in various cancer cell lines.. 2. Annonacin induces growth arrest and apoptosis in ERα-related pathways in MCF-7 cells, attenuates MCF-7 xenograft tumor growth while inhibiting ERα, cyclin D1 and Bcl-2 protein expressions in nude mice. 3. Annonacin-induced ATP depletion causes the retrograde transport of mitochondria to the cell soma and induces changes in the intracellular distribution of tau in a way that shares characteristics with some neurodegenerative diseases. |
| Targets | CDK | P21 | ERK | JNK | STAT | Bcl-2/Bax | Caspase | Estrogen receptor | Progestogen receptor |

Annonacin Dilution Calculator

Annonacin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6754 mL | 8.3768 mL | 16.7535 mL | 33.507 mL | 41.8838 mL |
| 5 mM | 0.3351 mL | 1.6754 mL | 3.3507 mL | 6.7014 mL | 8.3768 mL |
| 10 mM | 0.1675 mL | 0.8377 mL | 1.6754 mL | 3.3507 mL | 4.1884 mL |
| 50 mM | 0.0335 mL | 0.1675 mL | 0.3351 mL | 0.6701 mL | 0.8377 mL |
| 100 mM | 0.0168 mL | 0.0838 mL | 0.1675 mL | 0.3351 mL | 0.4188 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ruthenium Red
Catalog No.:BCC7067
CAS No.:11103-72-3
- Vitamin A2
Catalog No.:BCC8367
CAS No.:11103-57-4
- Pioglitazone
Catalog No.:BCC4927
CAS No.:111025-46-8
- Muricatide
Catalog No.:BCN1780
CAS No.:111025-01-5
- 2-Amino-1-phenylethanol
Catalog No.:BCN1779
CAS No.:7568-93-6
- Efonidipine hydrochloride monoethanolate
Catalog No.:BCC7767
CAS No.:111011-76-8
- (-)-Dihydroquercetin
Catalog No.:BCN3370
CAS No.:111003-33-9
- Deacetylsalannin
Catalog No.:BCN4733
CAS No.:1110-56-1
- Methyl Laurate
Catalog No.:BCC8211
CAS No.:111-82-0
- 1-Heptylamine
Catalog No.:BCN1801
CAS No.:111-68-2
- Oleylethanolamide
Catalog No.:BCC7084
CAS No.:111-58-0
- Diethanolamine
Catalog No.:BCN1797
CAS No.:111-42-2
- 2-(2'-Hydroxy-4'-methylphenyl)propionic acid
Catalog No.:BCN7980
CAS No.:111044-84-9
- Ginkgolic acid C17:1
Catalog No.:BCN5334
CAS No.:111047-30-4
- N-Benzoyl-2-hydroxy-2-phenylethylamine
Catalog No.:BCN1622
CAS No.:111059-46-2
- Fmoc-D-Lys(Trt)-OH
Catalog No.:BCC2594
CAS No.:111061-54-2
- Fmoc-Ser(Trt)-OH
Catalog No.:BCC3546
CAS No.:111061-56-4
- Dacomitinib (PF299804, PF299)
Catalog No.:BCC3683
CAS No.:1110813-31-4
- FERb 033
Catalog No.:BCC7701
CAS No.:1111084-78-6
- 14-Hydroxy sprengerinin C
Catalog No.:BCN2777
CAS No.:1111088-89-1
- NF 110
Catalog No.:BCC7404
CAS No.:111150-22-2
- PF-04880594
Catalog No.:BCC3998
CAS No.:1111636-35-1
- 1,2-O-Dilinoleoyl-3-O-beta-D-galactopyranosylracglycerol
Catalog No.:BCN6768
CAS No.:111187-15-6
- 7,3',4'-Trihydroxy-3-benzyl-2H-chromene
Catalog No.:BCN1621
CAS No.:1111897-60-9
Annonacin, a mono-tetrahydrofuran acetogenin, arrests cancer cells at the G1 phase and causes cytotoxicity in a Bax- and caspase-3-related pathway.[Pubmed:12697268]
Life Sci. 2003 May 9;72(25):2853-61.
Annonaceous acetogenins are a group of potential anti-neoplastic agents isolated from Annonaceae plants. In this study, we purified Annonacin, a cytotoxic mono-tetrahydrofuran acetogenin, from the seeds of Annona reticulata and analyzed its biological effects. Herein, we have shown that Annonacin caused significant cell death in various cancer cell lines. T24 bladder cancer cells at the S phase were more vulnerable to the cytotoxicity of Annonacin. Furthermore, Annonacin activated p21 in a p53-independent manner and arrested T24 cells at the G1 phase. It also induced Bax expression, enhanced caspase-3 activity, and caused apoptotic cell death in T24 cells. In summary, these results suggest that Annonacin is potentially a promising anti-cancer compound.
Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons.[Pubmed:17634376]
J Neurosci. 2007 Jul 18;27(29):7827-37.
A neurodegenerative tauopathy endemic to the Caribbean island of Guadeloupe has been associated with the consumption of anonaceous plants that contain acetogenins, potent lipophilic inhibitors of complex I of the mitochondrial respiratory chain. To test the hypothesis that Annonacin, a prototypical acetogenin, contributes to the etiology of the disease, we investigated whether Annonacin affects the cellular distribution of the protein tau. In primary cultures of rat striatal neurons treated for 48 h with Annonacin, there was a concentration-dependent decrease in ATP levels, a redistribution of tau from the axons to the cell body, and cell death. Annonacin induced the retrograde transport of mitochondria, some of which had tau attached to their outer membrane. Taxol, a drug that displaces tau from microtubules, prevented the somatic redistribution of both mitochondria and tau but not cell death. Antioxidants, which scavenged the reactive oxygen species produced by complex I inhibition, did not affect either the redistribution of tau or cell death. Both were prevented, however, by forced expression of the NDI1 nicotinamide adenine dinucleotide (NADH)-quinone-oxidoreductase of Saccharomyces cerevisiae, which can restore NADH oxidation in complex I-deficient mammalian cells and stimulation of energy production via anaerobic glycolysis. Consistently, other ATP-depleting neurotoxins (1-methyl-4-phenylpyridinium, 3-nitropropionic, and carbonyl cyanide m-chlorophenylhydrazone) reproduced the somatic redistribution of tau, whereas toxins that did not decrease ATP levels did not cause the redistribution of tau. Therefore, the Annonacin-induced ATP depletion causes the retrograde transport of mitochondria to the cell soma and induces changes in the intracellular distribution of tau in a way that shares characteristics with some neurodegenerative diseases.
Annonacin in Asimina triloba fruit: implication for neurotoxicity.[Pubmed:22130466]
Neurotoxicology. 2012 Jan;33(1):53-8.
INTRODUCTION: The acetogenin, Annonacin, from the tropical annonaceous plant Annona muricata, is a lipophilic, mitochondrial complex I inhibitor reported to be more toxic than rotenone to mesencephalic neurons. The temperate annonaceous plant Asimina triloba (pawpaw) is native to the Eastern United States and products are available online. This study determined whether Annonacin is in the pawpaw fruit pulp and whether it or the crude ethyl acetate extract is toxic to cortical neurons. METHODS: Pawpaw extract was prepared by pulp extraction with methanol and liquid-liquid partitioning with ethyl acetate (EtOAc). Annonacin was isolated from the crude EtOAc extract via column chromatography using a gradient solvent system of increasing polarity. Mass spectroscopy, nuclear magnetic resonance and infrared spectroscopy were used to compare isolated material with synthetic Annonacin data and a natural Annonacin sample. Toxicity of isolated Annonacin and the total EtOAc extract was determined in primary rat cortical neurons using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. RESULTS: The average concentration of Annonacin in the fruit pulp was 0.0701+/-0.0305mg/g. Purified Annonacin (30.07mug/ml) and crude EtOAc extract (47.96mug/ml) induced 50% death of cortical neurons 48h post treatment. Annonacin toxicity was enhanced in the presence of crude extract. DISCUSSION: Pawpaw fruit contains a high concentration of Annonacin, which is toxic to cortical neurons. Crude fruit extract also induced neurotoxicity, highlighting the need for additional studies to determine the potential risks of neurodegeneration associated with chronic exposure to pawpaw products.
Annonacin, a natural lipophilic mitochondrial complex I inhibitor, increases phosphorylation of tau in the brain of FTDP-17 transgenic mice.[Pubmed:24389273]
Exp Neurol. 2014 Mar;253:113-25.
Both genetic and environmental factors likely contribute to the neuropathology of tauopathies, but it remains unclear how specific genetic backgrounds affect the susceptibility towards environmental toxins. Mutations in the tau gene have been associated with familial tauopathies, while Annonacin, a plant-derived mitochondrial inhibitor, has been implicated in an environmental form of tauopathy. We therefore determined whether there was a pathogenic synergy between Annonacin exposure and the expression of the R406W-tau mutation in transgenic mice. We found that Annonacin exposure caused an increase in the number of neurons with phosphorylated tau in the somatodendritic compartment in several brain areas in R406W(+/+) mice as opposed to mice that had only the endogenous mouse tau (R406W(-/-)). Western blot analysis demonstrated a concomitant increase in total tau protein without increase in tau mRNA, but reduced proteasomal proteolytic activity in R406W(+/+), but not R406W(-/-) mice, upon Annonacin-treatment. Phosphorylated tau levels exceeded the increase in total tau protein, along with increased levels of different tau kinases, foremost a striking increase in the p25/p35 ratio, known to activate the tau kinase Cdk5. In summary, we observed a synergistic interaction between Annonacin exposure and the presence of the R406W-tau mutation, which resulted in reduced degradation, increased phosphorylation and redistribution of neuronal tau.
Annonacin induces cell cycle-dependent growth arrest and apoptosis in estrogen receptor-alpha-related pathways in MCF-7 cells.[Pubmed:21840388]
J Ethnopharmacol. 2011 Oct 11;137(3):1283-90.
ETHNOPHARMACOLOGICAL RELEVANCE: Tamoxifen resistance is common in estrogen receptor-alpha (ERalpha)-positive breast cancers. Pawpaw and soursop are anticancer annonaceous plants in complementary medicine. Thus, we studied the effects of Annonacin, an annonaceous acetogenin, in breast cancer cells. MATERIALS AND METHODS: Cell growth and ERalpha-related pathways were studied. The effects of Annonacin were tested in MCF-7 xenografts in nude mice. RESULTS: In ERalpha-positive MCF-7 cells, Annonacin (half-effective dose ED(50) = 0.31 muM) and 4-hydroxytamoxifen (ED(50) = 1.13 muM) decreased cell survival whereas Annonacin (0.5-1 muM) increased cell death at 48 h. Annonacin and 4-hydroxytamoxifen were additive in inhibiting cell survival. Annonacin (0.1 muM) induced G(0)/G(1) growth arrest while increasing p21(WAF1) and p27(kip1) and decreasing cyclin D1 protein expression. Annonacin (0.1muM) decreased cyclin D1 protein expression more than 4-hydroxytamoxifen (1 muM). Annonacin (0.1 muM) increased apoptosis while decreasing Bcl-2 protein expression. The combination of Annonacin (0.1 muM) and 4-hydroxytamoxifen (1 muM) decreased Bcl-2 protein expression and ERalpha transcriptional activity more than Annonacin (0.1 muM) did alone. Annonacin, but not 4-hydroxytamoxifen, decreased ERalpha protein expression. Moreover, Annonacin decreased phosphorylation of ERK1/2, JNK and STAT3. In nude mice, Annonacin decreased MCF-7 xenograft tumor size at 7-22 days. Moreover, Annonacin decreased ERalpha, cyclin D1 and Bcl-2 protein expression in the xenograft at 22 days. CONCLUSIONS: Annonacin induced growth arrest and apoptosis in ERalpha-related pathways in MCF-7 cells. Annonacin and 4-hydroxytamoxifen were additive in inhibiting cell survival and ERalpha transcriptional activity. Moreover, Annonacin attenuated MCF-7 xenograft tumor growth while inhibiting ERalpha, cyclin D1 and Bcl-2 protein expressions in nude mice.


