Allura Red ACCAS# 25956-17-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 25956-17-6 | SDF | Download SDF |
| PubChem ID | 33258 | Appearance | Red powder |
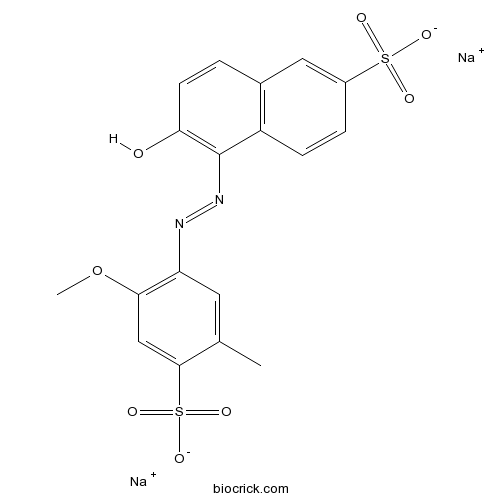
| Formula | C18H14N2Na2O8S2 | M.Wt | 496.42 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | disodium;6-hydroxy-5-[(2-methoxy-5-methyl-4-sulfonatophenyl)diazenyl]naphthalene-2-sulfonate | ||
| SMILES | CC1=CC(=C(C=C1S(=O)(=O)[O-])OC)N=NC2=C(C=CC3=C2C=CC(=C3)S(=O)(=O)[O-])O.[Na+].[Na+] | ||
| Standard InChIKey | CEZCCHQBSQPRMU-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/C18H16N2O8S2.2Na/c1-10-7-14(16(28-2)9-17(10)30(25,26)27)19-20-18-13-5-4-12(29(22,23)24)8-11(13)3-6-15(18)21;;/h3-9,21H,1-2H3,(H,22,23,24)(H,25,26,27);;/q;2*+1/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Allura Red AC and amaranth are very important food azo dyes used in food, drug, paper, cosmetic and textile industries. |
| Targets | Androgen Receptor | Estrogen receptor | Progestogen receptor |
| In vitro | Decolorization and mineralization of Allura Red AC aqueous solutions by electrochemical advanced oxidation processes.[Pubmed: 25734532]J Hazard Mater. 2015 Jun 15;290:34-42.The decolorization and mineralization of solutions containing 230 mg L(-1) of the food azo dye Allura Red AC at pH 3.0 have been studied upon treatment by electrochemical oxidation with electrogenerated H2O2 (EO-H2O2), electro-Fenton (EF) and photoelectro-Fenton (PEF). Genotoxicity assessment of amaranth and allura red using Saccharomyces cerevisiae.[Pubmed: 23132362]Bull Environ Contam Toxicol. 2013 Jan;90(1):22-6.Amaranth (E123) and Allura Red AC (E129), very important food azo dyes used in food, drug, paper, cosmetic and textile industries, were assessed for their genotoxic potential through comet assay in yeast cells. Comet assay was standardized by with different concentration of H(2)O(2). |
| In vivo | The synthetic food colouring agent Allura Red AC (E129) is not genotoxic in a flow cytometry-based micronucleus assay in vivo.[Pubmed: 23748052]Food Chem Toxicol. 2013 Sep;59:86-9.The safety of several azo colouring agents, used as food additives, has during the years been questioned. Allura Red AC (E129) has in some publications been classified as genotoxic. In fact, in the European Union, Allura Red AC is permitted as a food additive in human food, but, surprisingly, it was not acceptable as an additive for use in animal feed. |
| Structure Identification | Food Chem. 2015 Mar 1;170:423-9.Characterisation of interaction between food colourant allura red AC and human serum albumin: multispectroscopic analyses and docking simulations.[Pubmed: 25306366]
|

Allura Red AC Dilution Calculator

Allura Red AC Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0144 mL | 10.0721 mL | 20.1442 mL | 40.2885 mL | 50.3606 mL |
| 5 mM | 0.4029 mL | 2.0144 mL | 4.0288 mL | 8.0577 mL | 10.0721 mL |
| 10 mM | 0.2014 mL | 1.0072 mL | 2.0144 mL | 4.0288 mL | 5.0361 mL |
| 50 mM | 0.0403 mL | 0.2014 mL | 0.4029 mL | 0.8058 mL | 1.0072 mL |
| 100 mM | 0.0201 mL | 0.1007 mL | 0.2014 mL | 0.4029 mL | 0.5036 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- EDC.HCl
Catalog No.:BCC2812
CAS No.:25952-53-8
- HOBt (anhydrous)
Catalog No.:BCC2816
CAS No.:2592-95-2
- Boc-Lys(Boc)-ONp
Catalog No.:BCC3414
CAS No.:2592-19-0
- Boc-Thr-OH
Catalog No.:BCC3449
CAS No.:2592-18-9
- 3'-Hydroxydehydroaglaiastatin
Catalog No.:BCN7725
CAS No.:259143-58-3
- D-Luciferin
Catalog No.:BCC6535
CAS No.:2591-17-5
- Yunaconitoline
Catalog No.:BCN6703
CAS No.:259099-25-7
- Hoechst 33258 analog
Catalog No.:BCC1624
CAS No.:258843-62-8
- Liensinine
Catalog No.:BCN6337
CAS No.:2586-96-1
- H-D-Cys(Trt)-OH
Catalog No.:BCC2914
CAS No.:25840-82-8
- Ghrelin (rat)
Catalog No.:BCC5767
CAS No.:258338-12-4
- IEM 1925 dihydrobromide
Catalog No.:BCC7885
CAS No.:258282-23-4
- 5-Acetyl-3-chloro-10,11-dihydro-5H-dibenz[b,f]azepine
Catalog No.:BCC8726
CAS No.:25961-11-9
- 5-Hydroxy-7-methoxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1472
CAS No.:259653-54-8
- 2-Deacetyltaxuspine X
Catalog No.:BCN7375
CAS No.:259678-73-4
- T 705
Catalog No.:BCC4130
CAS No.:259793-96-9
- Hyponine D
Catalog No.:BCC8998
CAS No.:259823-31-9
- 4-Hydroxythiobenzamide
Catalog No.:BCC8709
CAS No.:25984-63-8
- Nimbolide
Catalog No.:BCN8053
CAS No.:25990-37-8
- Xylose
Catalog No.:BCC4880
CAS No.:25990-60-7
- GI 254023X
Catalog No.:BCC2374
CAS No.:260264-93-5
- Zamanic acid
Catalog No.:BCN5131
CAS No.:260393-05-3
- Dihydroisotanshinone II
Catalog No.:BCN5132
CAS No.:260397-58-8
- Ligustroflavone
Catalog No.:BCN2370
CAS No.:260413-62-5
The synthetic food colouring agent Allura Red AC (E129) is not genotoxic in a flow cytometry-based micronucleus assay in vivo.[Pubmed:23748052]
Food Chem Toxicol. 2013 Sep;59:86-9.
The safety of several azo colouring agents, used as food additives, has during the years been questioned. Allura Red AC (E129) has in some publications been classified as genotoxic. In fact, in the European Union, Allura Red is permitted as a food additive in human food, but, surprisingly, it was not acceptable as an additive for use in animal feed. In this study we have evaluated whether Allura Red is genotoxic using a flow cytometer-based micronucleus assay in peripheral blood of mice. Male FVB mice were given a single intra-peritoneal injection of various doses of Allura Red and sacrificed at 46 h after treatment. The tested doses were 0, 100, 200, 400, 600, 800, 1000, 1500, and 2000 mg/kg body weight (b.w.). Each dose group constituted three mice, except for in the dose group of 1000 mg/kg b. w., which constituted four mice. Blood samples were collected and the frequency of micronucleated polychromatic erythrocytes (fMNPCE) and the cell proliferation (%PCE) was determined. The analyses did not show any significant difference in the %PCE or in the fMNPCE. Consequently, under the testing circumstances one can conclude that Allura Red is not genotoxic.
Genotoxicity assessment of amaranth and allura red using Saccharomyces cerevisiae.[Pubmed:23132362]
Bull Environ Contam Toxicol. 2013 Jan;90(1):22-6.
Amaranth (E123) and Allura red (E129), very important food azo dyes used in food, drug, paper, cosmetic and textile industries, were assessed for their genotoxic potential through comet assay in yeast cells. Comet assay was standardized by with different concentration of H(2)O(2). Concentrations of Amaranth and Allura red were maintained in sorbitol buffer starting from 9.76 to 5,000 mug/mL and 1 x 10(4) cells were incubated at two different incubation temperatures 28 and 37 degrees C. Amaranth (E123) and Allura red (E129) were found to exhibit their genotoxic effect directly in Saccharomyces cerevisiae. No significant genotoxic activity was observed for Amaranth and Allura red at 28 degrees C but at 37 degrees C direct relation of Amaranth concentration with comet tail was significant and no positive relation was seen with time exposure factor. At 37 degrees C the minimum concentration of Amaranth and Allura red at which significant DNA damage observed through comet assay was 1,250 mug/mL in 2nd h post exposure time. The results indicated that food colors should be carefully used in baking products as heavy concentration of food colors could affect the fermentation process of baking.
Decolorization and mineralization of Allura Red AC aqueous solutions by electrochemical advanced oxidation processes.[Pubmed:25734532]
J Hazard Mater. 2015 Jun 15;290:34-42.
The decolorization and mineralization of solutions containing 230 mg L(-1) of the food azo dye Allura Red AC at pH 3.0 have been studied upon treatment by electrochemical oxidation with electrogenerated H2O2 (EO-H2O2), electro-Fenton (EF) and photoelectro-Fenton (PEF). Experiments were performed with a stirred tank reactor containing a boron-doped diamond (BDD) or Pt anode and an air-diffusion cathode to generate H2O2. The main oxidants were hydroxyl radicals formed at the anode surface from water oxidation and in the bulk from Fenton's reaction between H2O2 and added Fe(2+). The oxidation ability increased in the sequence EO-H2O2 < EF < PEF and faster degradation was always obtained using BDD. PEF process with BDD yielded almost total mineralization following similar trends in SO4(2-), ClO4(-) and NO3(-) media, whereas in Cl(-) medium, mineralization was inhibited by the formation of recalcitrant chloroderivatives. GC-MS analysis confirmed the cleavage of the -N=N- bond with formation of two main aromatics in SO4(2-) medium and three chloroaromatics in Cl(-) solutions. The effective oxidation of final oxalic and oxamic acids by BDD along with the photolysis of Fe(III)-oxalate species by UVA light accounted for the superiority of PEF with BDD. NH4(+), NO3(-) and SO4(2-) ions were released during the mineralization.
Characterisation of interaction between food colourant allura red AC and human serum albumin: multispectroscopic analyses and docking simulations.[Pubmed:25306366]
Food Chem. 2015 Mar 1;170:423-9.
Binding interaction of human serum albumin (HSA) with Allura Red AC, a food colourant, was investigated at the molecular level through fluorescence, ultraviolet-visible, circular dichroism (CD) and Raman spectroscopies, as well as protein-ligand docking studies to better understand the chemical absorption, distribution and transportation of colourants. Results show that Allura Red AC has the ability to quench the intrinsic fluorescence of HSA through static quenching. The negative values of the thermodynamic parameters DeltaG, DeltaH, and DeltaS indicated that hydrogen bond and van der Waals forces are dominant in the binding between the food colourant and HSA. The CD and Raman spectra showed that the binding of Allura Red AC to HSA induces the rearrangement of the carbonyl hydrogen-bonding network of polypeptides, which changes the HSA secondary structure. This colourant is bound to HSA in site I, and the binding mode was further analysed with the use of the CDOCKER algorithm in Discovery Studio.


