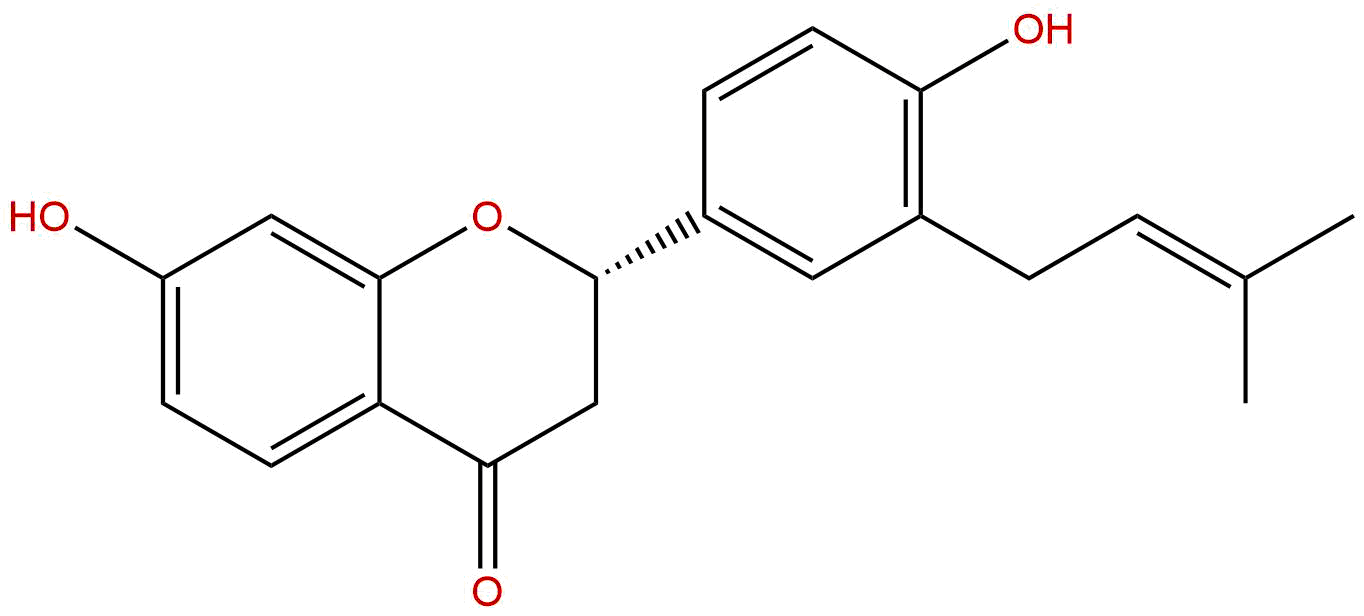
Abyssinone IICAS# 77263-08-2 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 77263-08-2 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C20H20O4 | M.Wt | 324.38 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Abyssinone II Dilution Calculator

Abyssinone II Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0828 mL | 15.414 mL | 30.828 mL | 61.6561 mL | 77.0701 mL |
| 5 mM | 0.6166 mL | 3.0828 mL | 6.1656 mL | 12.3312 mL | 15.414 mL |
| 10 mM | 0.3083 mL | 1.5414 mL | 3.0828 mL | 6.1656 mL | 7.707 mL |
| 50 mM | 0.0617 mL | 0.3083 mL | 0.6166 mL | 1.2331 mL | 1.5414 mL |
| 100 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6166 mL | 0.7707 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sphingomyelin
Catalog No.:BCX1119
CAS No.:6254-89-3
- 22,23-Dihydroergosterol
Catalog No.:BCX1118
CAS No.:516-79-0
- Inflacoumarin A
Catalog No.:BCX1117
CAS No.:158446-33-4
- Strigolactone
Catalog No.:BCX1116
CAS No.:76974-79-3
- Steviol-13-O-Glucoside
Catalog No.:BCX1115
CAS No.:60129-60-4
- Nifedipine impurity B
Catalog No.:BCX1114
CAS No.:50428-14-3
- Irisolidone 7-O-glucoside
Catalog No.:BCX1113
CAS No.:126308-74-5
- Maytansinol
Catalog No.:BCX1112
CAS No.:57103-68-1
- Maltooctaose
Catalog No.:BCX1111
CAS No.:66567-45-1
- Benzoyltropein
Catalog No.:BCX1110
CAS No.:19145-60-9
- Hyperforin acetate
Catalog No.:BCX1109
CAS No.:68324-06-1
- 4-(Acetyloxy)benzeneethanol
Catalog No.:BCX1108
CAS No.:60037-43-6
- Orchioside B
Catalog No.:BCX1121
CAS No.:851780-22-8
- Beta-Tomatine
Catalog No.:BCX1122
CAS No.:17406-46-1
- Monensin B
Catalog No.:BCX1123
CAS No.:30485-16-6
- Jaligonic acid B
Catalog No.:BCX1124
CAS No.:2375176-78-4
- Zymosterol
Catalog No.:BCX1125
CAS No.:128-33-6
- (-)-Epitaxifolin
Catalog No.:BCX1126
CAS No.:114761-89-6
- 2,3,4,6-tetraacetate Salidroside
Catalog No.:BCX1127
CAS No.:28251-63-0
- Salidroside pentaacetate
Catalog No.:BCX1128
CAS No.:39032-08-1
- Agigenin
Catalog No.:BCX1129
CAS No.:55332-76-8
- Ostruthine
Catalog No.:BCX1130
CAS No.:148-83-4
- L-Guluronic Acid Sodium Salt
Catalog No.:BCX1131
CAS No.:15769-56-9
- Tetraacetylphytosphingosine
Catalog No.:BCX1132
CAS No.:13018-48-9
Flavonoids as potential KRAS inhibitors: DFT, molecular docking, molecular dynamics simulation and ADMET analyses.[Pubmed:38647682]
J Asian Nat Prod Res. 2024 Apr 22:1-38.
KRAS mutations linked with cancer. Flavonoids were docked against KRAS G12C and G12D receptors. Abyssinone III, alpha naphthoflavone, beta naphthoflavone, abyssinone I, Abyssinone II and beta naphthoflavone, genistin, daidzin showed good docking scores against KRAS G12C and G12D receptors, respectively. The MD simulation data revealed that Rg, RMSD, RMSF, and SASA values were within acceptable limits. Alpha and beta naphthoflavone showed good binding energies with KRAS G12C and G12D receptors. DFT and MEP analysis highlighted the nucleophilic and electrophilic zones of best-docked flavonoids. A novel avenue for the control of KRAS G12C and G12D mutations is made possible by flavonoids.
Luteolin and abyssinone II as potential inhibitors of SARS-CoV-2: an in silico molecular modeling approach in battling the COVID-19 outbreak.[Pubmed:33495684]
Bull Natl Res Cent. 2021;45(1):27.
BACKGROUND: At present, the entire world is in a war against COVID-19 pandemic which has gradually led us toward a more compromised "new normal" life. SARS-CoV-2, the pathogenic microorganism liable for the recent COVID-19 outbreak, is extremely contagious in nature resulting in an unusual number of infections and death globally. The lack of clinically proven therapeutic intervention for COVID-19 has dragged the world's healthcare system into the biggest challenge. Therefore, development of an efficient treatment scheme is now in great demand. Screening of different biologically active plant-based natural compounds could be a useful strategy for combating this pandemic. In the present research, a collection of 43 flavonoids of 7 different classes with previously recorded antiviral activity was evaluated via computational and bioinformatics tools for their impeding capacity against SARS-CoV-2. In silico drug likeness, pharmacophore and Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) profile analysis of the finest ligands were carried out using DataWarrior, DruLiTo and admetSAR programs, respectively. Molecular docking was executed by AutoDock Vina, while molecular dynamics simulation of the target protein-ligand bound complexes was done using nanoscalable molecular dynamics and visual molecular dynamics software package. Finally, the molecular target analysis of the selected ligands within Homo sapiens was conducted with SwissTargetPredcition web server. RESULTS: Out of the forty-three flavonoids, luteolin and Abyssinone II were found to develop successful docked complex within the binding sites of target proteins in terms of lowest binding free energy and inhibition constant. The root mean square deviation and root mean square fluctuation values of the docked complex displayed stable interaction and efficient binding between the ligands and target proteins. Both of the flavonoids were found to be safe for human use and possessed good drug likeness properties and target accuracy. CONCLUSIONS: Conclusively, the current study proposes that luteolin and Abyssinone II might act as potential therapeutic candidates for SARS-CoV-2 infection. In vivo and in vitro experiments, however, should be taken under consideration to determine the efficiency and to demonstrate the mechanism of action.
Synthesis, structure-activity relationship studies, and antibacterial evaluation of 4-chromanones and chalcones, as well as olympicin A and derivatives.[Pubmed:25238443]
J Med Chem. 2014 Oct 23;57(20):8398-420.
On the basis of recently reported Abyssinone II and olympicin A, a series of chemically modified flavonoid phytochemicals were synthesized and evaluated against Mycobacterium tuberculosis and a panel of Gram-positive and -negative bacterial pathogens. Some of the synthesized compounds exhibited good antibacterial activities against Gram-positive pathogens including methicillin resistant Staphylococcus aureus with minimum inhibitory concentration as low as 0.39 mug/mL. SAR analysis revealed that the 2-hydrophobic substituent and the 4-hydrogen bond donor/acceptor of the 4-chromanone scaffold together with the hydroxy groups at 5- and 7-positions enhanced antibacterial activities; the 2',4'-dihydroxylated A ring and the lipophilic substituted B ring of chalcone derivatives were pharmacophoric elements for antibacterial activities. Mode of action studies performed on selected compounds revealed that they dissipated the bacterial membrane potential, resulting in the inhibition of macromolecular biosynthesis; further studies showed that selected compounds inhibited DNA topoisomerase IV, suggesting complex mechanisms of actions for compounds in this series.
Evaluation of flavonoid and resveratrol chemical libraries reveals abyssinone II as a promising antibacterial lead.[Pubmed:22847956]
ChemMedChem. 2012 Sep;7(9):1541-5.
Lead on! In the course of screening flavonoid and resveratrol libraries, Abyssinone II, a naturally occurring prenylated flavonoid, was found to exhibit relatively good antitubercular and antibacterial activity. Preliminary mechanistic studies revealed that Abyssinone II hyperpolarizes the bacterial membrane potential and inhibits the biosynthesis of key cellular macromolecules (DNA, RNA, and protein).
Prenylated flavonoid derivatives from the bark of Erythrina addisoniae.[Pubmed:18302333]
J Nat Prod. 2008 Apr;71(4):735-8.
Two new prenylated flavanones, 2 S-3'-(2-hydroxy-3-methylbut-3-enyl)licoflavone-4'-methyl ether ( 3) and 2 S-3'-(2-hydroxy-3-methylbut-3-enyl)Abyssinone II ( 4), and four known flavanones ( 1, 2, 5, 6) were isolated from the stem bark of Erythrina addisoniae. The structures were elucidated on the basis of their spectroscopic and physicochemical data. None of the compounds showed antioxidative properties. 4'-Methylabyssinone V ( 1) and abyssinoflavanone VII ( 6) showed moderate cytotoxic activity (IC 50 = 5 and 3.5 micromol/L, respectively), but apoptosis (caspase-3/7-activation, nuclear fragmentation) was selectively induced by abyssinoflavanone VII ( 6).
Synthesis and biological evaluation of (+/-)-abyssinone II and its analogues as aromatase inhibitors for chemoprevention of breast cancer.[Pubmed:17511439]
J Med Chem. 2007 Jun 14;50(12):2799-806.
An efficient and economical synthesis of the naturally occurring aromatase inhibitor Abyssinone II was performed. The synthesis features an optimized aromatic prenylation reaction in which an arylcopper intermediate is reacted with prenyl bromide to afford a key intermediate that was converted to a prenylated aromatic aldehyde. Condensation of the aldehyde with an o-hydroxyacetophenone under Claisen-Schmidt conditions afforded a chalcone that was deprotected and cyclized in the presence of sodium acetate in refluxing ethanol to afford (+/-)-Abyssinone II. The synthesis proved to be versatile enough to provide an array of Abyssinone II derivatives that were evaluated as aromatase inhibitors. Methylation of the 4'-hydroxyl group of (+/-)-Abyssinone II resulted in a significant increase in aromatase inhibitory activity, and further smaller increases in activity resulted from the methylation of the 7-hydroxyl group and removal of the prenyl side chain. As a result of these structural changes, the most active flavanone of the series was 20 times more potent than (+/-)-Abyssinone II (IC50 40.95 microM).
Synthesis of Abyssinone II and related compounds as potential chemopreventive agents.[Pubmed:16330130]
Eur J Med Chem. 2006 Feb;41(2):263-7.
A facile and efficient approach to the synthesis of prenylated flavonoids as potential chemopreventive agents has been described. This features the synthesis of prenyl halide, prenylation of p-hydroxybenzaldehyde, formation of prenylated polyhydroxychalcone and cyclization of prenylated polyhydroxychalcone to flavanones (15) and (16), and flavonol (17) starting from isoprene (1). The structures of all three compounds have been characterized by NMR, IR and mass spectroscopy.
Natural inhibitors of carcinogenesis.[Pubmed:15326546]
Planta Med. 2004 Aug;70(8):691-705.
Previous collaborative work by our group has led to the discovery of several plant isolates and derivatives with activities in in vivo models of cancer chemoprevention, including deguelin, resveratrol, bruceantin, brassinin, 4'-bromoflavone, and oxomate. Using a panel of in vitro bioassays to monitor chromatographic fractionation, a diverse group of plant secondary metabolites has been identified as potential cancer chemopreventive agents from mainly edible plants. Nearly 50 new compounds have been isolated as bioactive principles in one or more in vitro bioassays in work performed over the last five years. Included among these new active compounds are alkaloids, flavonoids, stilbenoids, and withanolides, as well as a novel stilbenolignan and the first representatives of the norwithanolides, which have a 27-carbon atom skeleton. In addition, over 100 active compounds of previously known structure have been obtained. Based on this large pool of potential cancer chemopreventive compounds, structure-activity relationships are discussed in terms of the quinone reductase induction ability of flavonoids and withanolides and the cyclooxygenase-1 and -2 inhibitory activities of flavanones, flavones and stilbenoids. Several of the bioactive compounds were found to be active when evaluated in a mouse mammary organ culture assay, when used as a secondary discriminator in our work. The compounds (2 S)-Abyssinone II, (2 S)-2',4'-dihydroxy-2"-(1-hydroxy-1-methylethyl)dihydrofuro[2,3- h]-flavanone, 3'-[gamma-hydroxymethyl-( E)-gamma-methylallyl]-2,4,2',4'-tetrahydroxychalcone 11'- O-coumarate, isolicoflavonol, isoliquiritigenin, and ixocarpalactone A are regarded as promising leads as potential cancer chemopreventive agents.
Aromatase inhibitors from Broussonetia papyrifera.[Pubmed:11678652]
J Nat Prod. 2001 Oct;64(10):1286-93.
Bioassay-guided fractionation of an ethyl acetate-soluble extract from the whole plants of Broussonetia papyrifera, using an in vitro aromatase inhibition assay, led to the isolation of five new active compounds, 5,7,2',4'-tetrahydroxy-3-geranylflavone (1), isogemichalcone C (8), 3'-[gamma-hydroxymethyl-(E)-gamma-methylallyl]-2,4,2',4'-tetrahydroxychalcone 11'-O-coumarate (9), demethylmoracin I (10), and (2S)-2',4'-dihydroxy-2' '-(1-hydroxy-1-methylethyl)dihydrofuro[2,3-h]flavanone (11), and 10 known (12-21) compounds which were also found to be active. Of these compounds, the most potent were 9 (IC(50) 0.5 microM), 11 (IC(50) 0.1 microM), isolicoflavonol (12, IC(50) 0.1 microM), and (2S)-Abyssinone II (13, IC(50) 0.4 microM). Additionally, six new compounds, 5,7,3',4'-tetrahydroxy-6-geranylflavonol (2), 5,7,3',4'-tetrahydroxy-3-methoxy-6-geranylflavone (3), (2S)-7,4'-dihydroxy-3'-prenylflavan (4), 1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)propane (5), 1-(2,4-dihydroxy-3-prenylphenyl)-3-(4-hydroxyphenyl)propane (6), and 1-(4-hydroxy-2-methoxyphenyl)-3-(4-hydroxy-3-prenylphenyl)propane (7), were isolated and characterized, but proved to be inactive as aromatase inhibitors, as were an additional 21 known compounds. The structures of the new compounds (1-11) were elucidated by spectroscopic methods. Structure-activity relationships in the aromatase assay were determined for the benzofurans, biphenylpropanoids, coumarins, and various types of flavonoids (chalcones, flavans, flavanones, and flavones) obtained among a total of 42 constituents of B. papyrifera.


