ARN-509Androgen receptor inhibitor CAS# 956104-40-8 |
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- RU 58841
Catalog No.:BCC1911
CAS No.:154992-24-2
- Andarine
Catalog No.:BCC1168
CAS No.:401900-40-1
- Spironolactone
Catalog No.:BCC4366
CAS No.:52-01-7
- TOK-001
Catalog No.:BCC3910
CAS No.:851983-85-2
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 956104-40-8 | SDF | Download SDF |
| PubChem ID | 24872560 | Appearance | Powder |
| Formula | C21H15F4N5O2S | M.Wt | 477.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Apalutamide | ||
| Solubility | DMSO : ≥ 83.3 mg/mL (174.48 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
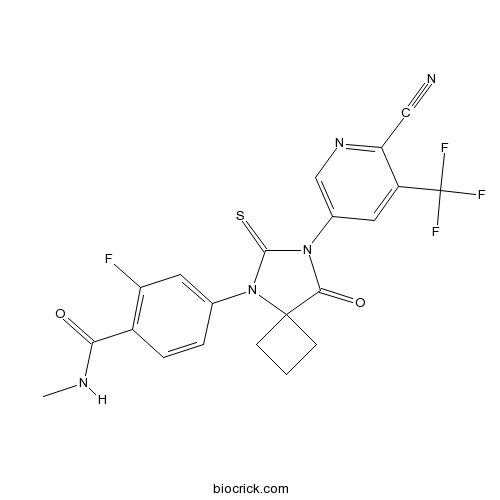
| Chemical Name | 4-[7-[6-cyano-5-(trifluoromethyl)pyridin-3-yl]-8-oxo-6-sulfanylidene-5,7-diazaspiro[3.4]octan-5-yl]-2-fluoro-N-methylbenzamide | ||
| SMILES | CNC(=O)C1=C(C=C(C=C1)N2C(=S)N(C(=O)C23CCC3)C4=CN=C(C(=C4)C(F)(F)F)C#N)F | ||
| Standard InChIKey | HJBWBFZLDZWPHF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H15F4N5O2S/c1-27-17(31)13-4-3-11(8-15(13)22)30-19(33)29(18(32)20(30)5-2-6-20)12-7-14(21(23,24)25)16(9-26)28-10-12/h3-4,7-8,10H,2,5-6H2,1H3,(H,27,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | ARN-509 is a selective and competitive inhibitor of androgen receptor with an IC50 value of 16 nM. | |||||
| Targets | Androgen Receptor | GABAA receptor | ||||
| IC50 | 16 nM | 3 μM | ||||

ARN-509 Dilution Calculator

ARN-509 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0945 mL | 10.4727 mL | 20.9455 mL | 41.891 mL | 52.3637 mL |
| 5 mM | 0.4189 mL | 2.0945 mL | 4.1891 mL | 8.3782 mL | 10.4727 mL |
| 10 mM | 0.2095 mL | 1.0473 mL | 2.0945 mL | 4.1891 mL | 5.2364 mL |
| 50 mM | 0.0419 mL | 0.2095 mL | 0.4189 mL | 0.8378 mL | 1.0473 mL |
| 100 mM | 0.0209 mL | 0.1047 mL | 0.2095 mL | 0.4189 mL | 0.5236 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ARN-509, a synthetic biaryl thiohydantoin compound, is a competitive androgen receptor (AR) inhibitor and fully antagonistic to AR overexpression. The IC50 of ARN-509 is 16 nmol/L [1].
AR, included in the steroid receptor superfamily, is important for prostate cell proliferation and male sexual differentiation [2]. AR overexpression is a common and important feature of castration resistant prostate cancer (CRPC) [1].
ARN-509 (1 μmol/L) treatment for 48 hours resulted in increased DNA damage in LNCaP cells, LNCaP-AR cells and VCaP cells. In LNCaP cell line, treatment with ARN-509 (1 μmol/L) resulted in decreased cell survival. Treatment with ARN-509 (1 μmol/L) for 48 hours significantly decreased C-NHEJ–mediated recombination (>60%) in LNCaP cells that had been transfected with V(D)J recombination substrate along with RAG1 and RAG2 expression vectors [3]. ARN-509 showed robust transcriptional and proliferative agonist activity in AR F876L–expressing cells, and promoted AR DNA binding in LNCaP/SRαF876L cells [4].
Orally treated with ARN-509 (10 mg/kg/d) for 17 days, androgendriven luciferase reporter–gene activity in castrate male immunodeficient mice harboring LNCaP/AR-luc xenograft tumors (coexpressing exogenous AR and the AR-dependent reporter ARR2-Pb-luc), was consistently reduced. This indicated that ARN-509 inhibited AR in vivo. ARN-509 made tumors exhibit a decrease in proliferative index and an increase in apoptotic rate [1].
References:
[1]. Nicola J. Clegg, John Wongvipat, James D. Joseph, et al. ARN-509: A Novel Antiandrogen for Prostate Cancer Treatment. Therapeutics, Targets & Chemical Biology, 2012, 72(6): 1494-1503.
[2]. Shuyuan Yeh and Chawnshang Chang. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc. Natl. Acad. Sci., 1996, 93: 5517-5521.
[3]. William R. Polkinghorn, Joel S. Parker, Man X. Lee, et al. Androgen Receptor Signaling Regulates DNA Repair in Prostate Cancers. Cancer Discovery, 2013, 3(11):1245-53.
[4]. James D. Joseph, Nhin Lu, Jing Qian, et al. A Clinically Relevant Androgen Receptor Mutation Confers Resistance to Second-Generation Antiandrogens Enzalutamide and ARN-509. Cancer Discovery, 2013, 3(9):1020-9.
- Cochinchinenin C
Catalog No.:BCN5329
CAS No.:956103-79-0
- (2R)-8-Methylsocotrin-4'-ol
Catalog No.:BCN3737
CAS No.:956103-75-6
- NS 11021
Catalog No.:BCC6290
CAS No.:956014-19-0
- PI-3065
Catalog No.:BCC5379
CAS No.:955977-50-1
- MK-1775
Catalog No.:BCC2543
CAS No.:955365-80-7
- 8,3'-Diprenylapigenin
Catalog No.:BCN6482
CAS No.:955135-37-2
- 10Panx
Catalog No.:BCC1245
CAS No.:955091-53-9
- 5-Hydroxy-7-acetoxy-8-methoxyflavone
Catalog No.:BCN4506
CAS No.:95480-80-1
- Hydroxytuberosone
Catalog No.:BCN4552
CAS No.:95456-43-2
- Neotuberostemonone
Catalog No.:BCN4505
CAS No.:954379-68-1
- 15-Hydroxy-7-oxodehydroabietic acid
Catalog No.:BCN4504
CAS No.:95416-25-4
- BLZ945
Catalog No.:BCC5583
CAS No.:953769-46-5
- LCQ-908
Catalog No.:BCC1692
CAS No.:956136-95-1
- TCS HDAC6 20b
Catalog No.:BCC2427
CAS No.:956154-63-5
- mavatrep
Catalog No.:BCC6457
CAS No.:956274-94-5
- Phoyunnanin C
Catalog No.:BCN3686
CAS No.:956344-38-0
- Ranolazine
Catalog No.:BCC3847
CAS No.:95635-55-5
- Ranolazine 2HCl
Catalog No.:BCC2503
CAS No.:95635-56-6
- Demethylsonchifolin
Catalog No.:BCN4551
CAS No.:956384-55-7
- UNBS 5162
Catalog No.:BCC4008
CAS No.:956590-23-1
- MM-22
Catalog No.:BCC6114
CAS No.:956605-71-3
- LDE225 (NVP-LDE225,Erismodegib)
Catalog No.:BCC5066
CAS No.:956697-53-3
- 8beta,9alpha-Dihydroxylindan-4(5),7(11)-dien-8alpha,12-olide
Catalog No.:BCN8024
CAS No.:956707-04-3
- Euscaphin B
Catalog No.:BCN4507
CAS No.:956869-95-7
Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer.[Pubmed:24002508]
J Clin Oncol. 2013 Oct 1;31(28):3525-30.
PURPOSE: ARN-509 is a novel androgen receptor (AR) antagonist for the treatment of castration-resistant prostate cancer (CRPC). ARN-509 inhibits AR nuclear translocation and AR binding to androgen response elements and, unlike bicalutamide, does not exhibit agonist properties in the context of AR overexpression. This first-in-human phase I study assessed safety, tolerability, pharmacokinetics, pharmacodynamics, and antitumor activity of ARN-509 in men with metastatic CRPC. PATIENTS AND METHODS: Thirty patients with progressive CRPC received continuous daily oral ARN-509 at doses between 30 and 480 mg, preceded by administration of a single dose followed by a 1-week observation period with pharmacokinetic sampling. Positron emission tomography/computed tomography imaging was conducted to monitor [(18)F]fluoro-alpha-dihydrotestosterone (FDHT) binding to AR in tumors before and during treatment. Primary objective was to determine pharmacokinetics, safety, and recommended phase II dose. RESULTS: Pharmacokinetics were linear and dose proportional. Prostate-specific antigen declines at 12 weeks (>/= 50% reduction from baseline) were observed in 46.7% of patients. Reduction in FDHT uptake was observed at all doses, with a plateau in response at >/= 120-mg dose, consistent with saturation of AR binding. The most frequently reported adverse event was grade 1/2 fatigue (47%). One dose-limiting toxicity event (grade 3 abdominal pain) occurred at the 300-mg dose. Dose escalation to 480 mg did not identify a maximum-tolerated dose. CONCLUSION: ARN-509 was safe and well tolerated, displayed dose-proportional pharmacokinetics, and demonstrated pharmacodynamic and antitumor activity across all dose levels tested. A maximum efficacious dose of 240 mg daily was selected for phase II exploration based on integration of preclinical and clinical data.
Phase 2 Study of the Safety and Antitumor Activity of Apalutamide (ARN-509), a Potent Androgen Receptor Antagonist, in the High-risk Nonmetastatic Castration-resistant Prostate Cancer Cohort.[Pubmed:27160947]
Eur Urol. 2016 Dec;70(6):963-970.
BACKGROUND: Apalutamide is a potent androgen receptor (AR) antagonist that targets the AR ligand-binding domain and prevents AR nuclear translocation, DNA binding, and transcription of AR gene targets. OBJECTIVE: To evaluate the activity and safety of apalutamide in patients with high-risk nonmetastatic castration-resistant prostate cancer (nmCRPC). DESIGN, SETTING, AND PARTICIPANTS: We conducted a multicenter phase 2 study of nmCRPC patients with a high risk for progression (prostate-specific antigen [PSA] >/=8 ng/ml or PSA doubling time [PSA DT] /=50% PSA decline at 12 wk. Median TTPP was 24.0 mo (95% confidence interval [CI], 16.3 mo-not reached [NR]); median MFS was NR (95% CI, 33.4 mo-NR). Most of the patients discontinued study treatment (n=33) due to disease progression (n=11 [22%]) or adverse events (AEs) (n=9 [18%]). The most common AE was fatigue (any grade, n=31 [61%]) although grade >/=3 fatigue was uncommon (n=2 [4%]). These represent the first apalutamide nmCRPC patient clinical data. CONCLUSIONS: In high-risk nmCRPC patients, apalutamide was safe with robust activity based on durable PSA responses and disease control. PATIENT SUMMARY: Antitumor activity and the safety of apalutamide in patients with nonmetastatic castration-resistant prostate cancer support continued development in this setting. TRIAL REGISTRATION: ClinicalTrials.gov identifier NCT01171898.
Safety and Antitumor Activity of Apalutamide (ARN-509) in Metastatic Castration-Resistant Prostate Cancer with and without Prior Abiraterone Acetate and Prednisone.[Pubmed:28213364]
Clin Cancer Res. 2017 Jul 15;23(14):3544-3551.
Purpose: To evaluate the efficacy of apalutamide before or after treatment with abiraterone acetate and prednisone (AAP) in patients with progressive metastatic castration-resistant prostate cancer (mCRPC).Experimental Design: Two cohorts were studied: AAP-naive and post-AAP patients who had received >/=6 months of AAP. Patients had progressive mCRPC per rising prostate-specific antigen (PSA) and/or imaging, without prior chemotherapy exposure. All received apalutamide 240 mg/day. Primary endpoint was >/=50% decline in 12-week PSA according to Prostate Cancer Working Group 2 criteria. Secondary endpoints included time to PSA progression and time on treatment.Results: Forty-six patients enrolled in the AAP-naive (n = 25) and post-AAP (n = 21) cohorts. The 12-week PSA response rate was 88% (22/25) and 22% (4/18), median time to PSA progression was 18.2 months [95% confidence interval (CI), 8.3 months-not reached) and 3.7 months (95% CI, 2.8-5.6 months), and median time on treatment 21 months (range, 2.6-37.5) and 4.9 months (range, 1.3-23.2), for the AAP-naive and post-AAP cohorts, respectively. Eighty percent (95% CI, 59-93) and 64% (95% CI, 43-82) of AAP-naive and 43% (95% CI, 22-66) and 10% (95% CI, 1-30) of post-AAP patients remained on treatment for 6+ and 12+ months, respectively. Common treatment-emergent adverse events in both cohorts were grade 1 or 2 fatigue, diarrhea, nausea, and abdominal pain.Conclusions: Apalutamide was safe, well tolerated, and demonstrated clinical activity in mCRPC, with 80% of AAP-naive and 43% of post-AAP patients, remaining on treatment for 6 months or longer. Clin Cancer Res; 23(14); 3544-51. (c)2017 AACR.


