AM-095 free basePotent LPA1 receptor antagonist CAS# 1228690-36-5 |
Quality Control & MSDS
Number of papers citing our products

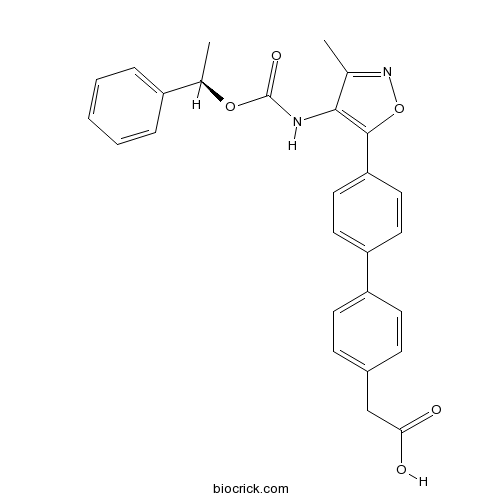
Chemical structure

3D structure
| Cas No. | 1228690-36-5 | SDF | Download SDF |
| PubChem ID | 46213949 | Appearance | Powder |
| Formula | C27H24N2O5 | M.Wt | 456.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 67.3 mg/mL (147.43 mM; Need ultrasonic and warming) | ||
| Chemical Name | 2-[4-[4-[3-methyl-4-[[(1R)-1-phenylethoxy]carbonylamino]-1,2-oxazol-5-yl]phenyl]phenyl]acetic acid | ||
| SMILES | CC1=NOC(=C1NC(=O)OC(C)C2=CC=CC=C2)C3=CC=C(C=C3)C4=CC=C(C=C4)CC(=O)O | ||
| Standard InChIKey | LNDDRUPAICPXIN-GOSISDBHSA-N | ||
| Standard InChI | InChI=1S/C27H24N2O5/c1-17-25(28-27(32)33-18(2)20-6-4-3-5-7-20)26(34-29-17)23-14-12-22(13-15-23)21-10-8-19(9-11-21)16-24(30)31/h3-15,18H,16H2,1-2H3,(H,28,32)(H,30,31)/t18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AM095 is a potent antagonist of LPA1 receptor with IC50 values of 0.98 and 0.73 μM for recombinant human and mouse LPA1, respectively. | |||||
| Targets | LPA1 receptor | |||||
| IC50 | 0.98 μM (recombinant human) 0.73 μM (recombinant mouse) | |||||

AM-095 free base Dilution Calculator

AM-095 free base Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1906 mL | 10.9531 mL | 21.9063 mL | 43.8126 mL | 54.7657 mL |
| 5 mM | 0.4381 mL | 2.1906 mL | 4.3813 mL | 8.7625 mL | 10.9531 mL |
| 10 mM | 0.2191 mL | 1.0953 mL | 2.1906 mL | 4.3813 mL | 5.4766 mL |
| 50 mM | 0.0438 mL | 0.2191 mL | 0.4381 mL | 0.8763 mL | 1.0953 mL |
| 100 mM | 0.0219 mL | 0.1095 mL | 0.2191 mL | 0.4381 mL | 0.5477 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AM095 is a novel, potent and orally bioavailable antagonist of lysophosphatidic acid type 1 receptor (LPA1) with IC50 values of 0.73 and 0.98 μM for mouse or recombinant human LPA1, respectively [1].
In vitro, AM095 has shown to inhibit LPA1-induced chemotaxis of both mouse LPA1/CHO cells and human A2058 melanoma cells with IC50 values of 0.78 μM and 0.23μM [1].
In vivo, AM095 could dose-dependently block LPA-induced histamine release with an ED50 value of 8.3 mg/kg in mice. Additionally, AM095 has been revealed to remarkably reduce the BALF collagen and protein with an ED50 value of 10 mg/kg in lungs. AM095 has also shown to decrease both macrophage and lymphocyte infiltration induced by bleomycin in mice [1].
References:
[1] Swaney JS1, Chapman C, Correa LD, Stebbins KJ, Broadhead AR, Bain G, Santini AM, Darlington J, King CD, Baccei CS, Lee C, Parr TA, Roppe JR, Seiders TJ, Ziff J, Prasit P, Hutchinson JH, Evans JF, Lorrain DS. Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J Pharmacol Exp Ther. 2011 Mar;336(3):693-700.
- AM966
Catalog No.:BCC1355
CAS No.:1228690-19-4
- TAK-632
Catalog No.:BCC3639
CAS No.:1228591-30-7
- GS-9620
Catalog No.:BCC1602
CAS No.:1228585-88-3
- Panaxyne
Catalog No.:BCN6462
CAS No.:122855-49-6
- Alosetron Hydrochloride
Catalog No.:BCC1344
CAS No.:122852-69-1
- Alosetron (Z)-2-butenedioate
Catalog No.:BCC1343
CAS No.:122852-43-1
- Alosetron
Catalog No.:BCC1342
CAS No.:122852-42-0
- Cefoselis Sulfate
Catalog No.:BCC4769
CAS No.:122841-12-7
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- H-D-Phe(4-F)-OH .HCl
Catalog No.:BCC3217
CAS No.:122839-52-5
- MRS 2957 triethylammonium salt
Catalog No.:BCC6133
CAS No.:1228271-30-4
- 8-Geranyloxy-5,7-dimethoxycoumarin
Catalog No.:BCN6117
CAS No.:1228175-65-2
- 2-Desoxy-4-epi-pulchellin
Catalog No.:BCN6118
CAS No.:122872-03-1
- Mps1-IN-2
Catalog No.:BCC4153
CAS No.:1228817-38-6
- Ziprasidone HCl
Catalog No.:BCC2511
CAS No.:122883-93-6
- MLN0905
Catalog No.:BCC3961
CAS No.:1228960-69-7
- 12-Demethylneocaesalpin F
Catalog No.:BCN6410
CAS No.:1228964-10-0
- Tatarinoid A
Catalog No.:BCN6119
CAS No.:1229005-35-9
- GSK 9027
Catalog No.:BCC6115
CAS No.:1229096-88-1
- 3-O-Acetylandrostenone hydrazone
Catalog No.:BCC8637
CAS No.:122914-94-7
- Edoxaban tosylate monohydrate
Catalog No.:BCC1545
CAS No.:1229194-11-9
- GS-9973
Catalog No.:BCC5278
CAS No.:1229208-44-9
- C 21
Catalog No.:BCC8013
CAS No.:1229236-78-5
- LY2784544
Catalog No.:BCC2200
CAS No.:1229236-86-5
Synthesis of Cyano-Containing Phenanthridine Derivatives via Catalyst-, Base-, and Oxidant-Free Direct Cyanoalkylarylation of Isocyanides.[Pubmed:28362091]
J Org Chem. 2017 Apr 21;82(8):4444-4448.
An efficient catalyst-, base-, and oxidant-free direct cyanoalkylarylation of isocyanides with AIBN has been developed under mild conditions. This strategy provides an elusive and rapid access to a wide range of cyano-containing phenanthridine derivatives in good yields via a one-pot alkylation/cyclization radical-cascade process. The mild reaction conditions together with no need of any catalyst, base, or oxidant make this protocol environmentally benign and practical.
Stabilization and Structure of the cis Tautomer of a Free-Base Porphyrin.[Pubmed:28370984]
Angew Chem Int Ed Engl. 2017 Aug 14;56(34):10088-10092.
Single-crystal X-ray analysis of the beta-heptakis(trifluoromethyl)-meso-tetrakis(p-fluorophenyl)porphyrin, H2 [(CF3 )7 TpFPP], has revealed the first example of a stable cis tautomer of a free-base porphyrin, the long-postulated intermediate of porphyrin tautomerism. The stability of the unique molecule appears to reflect a dual origin: a strongly saddled porphyrin skeleton, which alleviates electrostatic repulsion between the two NH protons, and two polarization-enhanced, transannular N-HO-HN hydrogen bond chains, each involving a molecule of water. DFT calculations suggest that the observed tautomer has a lower energy than the alternative, doubly hydrated trans tautomer by some 8.3 kcal mol(-1) . A fascinating prospect thus exists that H2 [(CF3 )7 TpFPP]2 H2 O and cognate structures may act as supramolecular synthons, which, given their chirality, may even be amenable to resolution into optically pure enantiomers.
The Laparoscopically Harvested Omental Free Flap: A Compelling Option for Craniofacial and Cranial Base Reconstruction.[Pubmed:28321385]
J Neurol Surg B Skull Base. 2017 Apr;78(2):191-196.
Background Management of craniofacial and cranial base tumors is a challenge due to the anatomic intricacies associated with the calvarium, the pathological diversity of lesions that present, and the potential complications. Clinical outcomes in laparoscopically harvested omentum free flaps for cranial base and craniofacial reconstruction are presented in this paper, in the largest case series to date. Methods A retrospective single-center experience for over 10 years with laparoscopically harvested omentum flaps used to reconstruct craniofacial and cranial base defects. Results A total of 13 patients underwent craniofacial or cranial base reconstruction using laparoscopically harvested omentum free flaps. The mean patient age was 48 years. The anterior skull base represented the most common site of reconstruction. A total of 12 of the flaps survived (92%), with one flap failure due to infection. All patients demonstrated satisfactory aesthetic and functional outcomes. There were no perioperative or intra-abdominal complications. Conclusions The laparoscopically harvested omentum free flap is a safe and effective tool in the armamentarium of the reconstructive surgeon. It is the ideal option to treat complex, three-dimensional subcutaneous defects, such as those encountered in craniofacial and cranial base reconstruction. Its unique angiogenic and immunologic capacity makes it an excellent flap for the previously irradiated and/or infected wound bed.
Chiral Tertiary Sulfonium Salts as Effective Catalysts for Asymmetric Base-Free Neutral Phase-Transfer Reactions.[Pubmed:28371093]
Angew Chem Int Ed Engl. 2017 Apr 18;56(17):4819-4823.
Although chiral quaternary ammonium and phosphonium salts are commonly used for asymmetric organocatalysis, the catalytic ability of chiral tertiary sulfonium salts has yet to be demonstrated in asymmetric synthesis. Herein, we show that chiral bifunctional trialkylsulfonium salts catalyze highly enantioselective conjugate additions of 3-substituted oxindoles to maleimides under base-free neutral phase-transfer conditions.


