ACETCAS# 936095-50-0 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 936095-50-0 | SDF | Download SDF |
| PubChem ID | 118797384 | Appearance | Powder |
| Formula | C20H19N3O6S | M.Wt | 429.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in 3eq. NaOH | ||
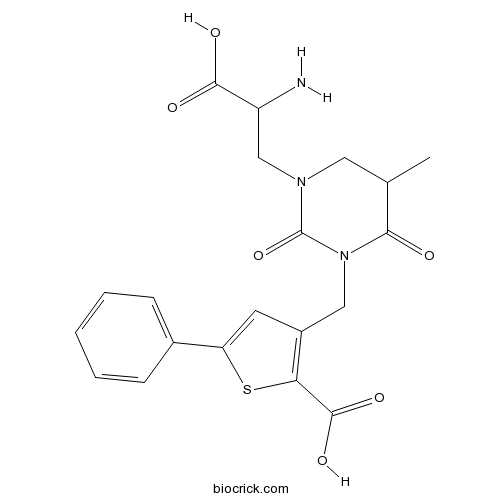
| Chemical Name | 3-[[3-(2-amino-2-carboxyethyl)-5-methyl-2,6-dioxo-1,3-diazinan-1-yl]methyl]-5-phenylthiophene-2-carboxylic acid | ||
| SMILES | CC1CN(C(=O)N(C1=O)CC2=C(SC(=C2)C3=CC=CC=C3)C(=O)O)CC(C(=O)O)N | ||
| Standard InChIKey | OAAMYRWMIRGBQF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H21N3O6S/c1-11-8-22(10-14(21)18(25)26)20(29)23(17(11)24)9-13-7-15(30-16(13)19(27)28)12-5-3-2-4-6-12/h2-7,11,14H,8-10,21H2,1H3,(H,25,26)(H,27,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective GluK1 (formerly GluR5) containing kainate receptor antagonist (IC50 = 7 nM) that displays selectivity over GluK2 (formerly GluR6) containing kainate, NMDA, AMPA and group I mGlu receptors. Reversibly blocks induction of NMDA receptor-independent long term potentiation (LTP) in vitro at nanomolar concentrations. |

ACET Dilution Calculator

ACET Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3286 mL | 11.6428 mL | 23.2856 mL | 46.5712 mL | 58.214 mL |
| 5 mM | 0.4657 mL | 2.3286 mL | 4.6571 mL | 9.3142 mL | 11.6428 mL |
| 10 mM | 0.2329 mL | 1.1643 mL | 2.3286 mL | 4.6571 mL | 5.8214 mL |
| 50 mM | 0.0466 mL | 0.2329 mL | 0.4657 mL | 0.9314 mL | 1.1643 mL |
| 100 mM | 0.0233 mL | 0.1164 mL | 0.2329 mL | 0.4657 mL | 0.5821 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- TG101348 (SAR302503)
Catalog No.:BCC2190
CAS No.:936091-26-8
- TG101209
Catalog No.:BCC2198
CAS No.:936091-14-4
- cAMPS-Sp, triethylammonium salt
Catalog No.:BCC8081
CAS No.:93602-66-5
- Daphnenone
Catalog No.:BCN3229
CAS No.:936006-13-2
- 5-(6-Hydroxybenzofuran-2-yl)-2-(3-methylbut-1-enyl)benzene-1,3-diol
Catalog No.:BCN1304
CAS No.:936006-11-0
- Oprozomib (ONX-0912)
Catalog No.:BCC1146
CAS No.:935888-69-0
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
- BIX 01294
Catalog No.:BCC1131
CAS No.:935693-62-2
- AZD1480
Catalog No.:BCC2191
CAS No.:935666-88-9
- MDL 72527
Catalog No.:BCC6060
CAS No.:93565-01-6
- Euphorbia factor L7a
Catalog No.:BCN3784
CAS No.:93550-94-8
- TH-237A
Catalog No.:BCC5378
CAS No.:935467-97-3
- Ajuganipponin A
Catalog No.:BCN3660
CAS No.:936323-13-6
- PCI-32765 Racemate
Catalog No.:BCC5124
CAS No.:936563-87-0
- PCI-32765 (Ibrutinib)
Catalog No.:BCC1266
CAS No.:936563-96-1
- LCZ696
Catalog No.:BCC5505
CAS No.:936623-90-4
- VX-809
Catalog No.:BCC3712
CAS No.:936727-05-8
- Magnolignan A
Catalog No.:BCN4084
CAS No.:93673-81-5
- Rengyol
Catalog No.:BCN4481
CAS No.:93675-85-5
- Forsythoside E
Catalog No.:BCN2782
CAS No.:93675-88-8
- OSI-027
Catalog No.:BCC4603
CAS No.:936890-98-1
- Magnolignan C
Catalog No.:BCN4085
CAS No.:93697-42-8
- TC-H 106
Catalog No.:BCC2426
CAS No.:937039-45-7
- Leuconolam
Catalog No.:BCN4482
CAS No.:93710-27-1
Crystal structure of 3-acet-oxy-2-methyl-benzoic acid.[Pubmed:26279915]
Acta Crystallogr E Crystallogr Commun. 2015 Jun 13;71(Pt 7):o474.
In the title mol-ecule, C10H10O4, the carb-oxy-lic acid group is twisted by 11.37 (15) degrees from the plane of the benzene ring and the ACET-oxy group is twisted from this plane by 86.60 (17) degrees . In the crystal, mol-ecules are linked by pairs of O-Hcdots, three dots, centeredO hydrogen bonds, forming inversion dimers with the expected R 2 (2)(8) graph-set motif.
Crystal structure of diethyl 2-acet-oxy-2-[3-(4-nitro-phen-yl)-3-oxo-1-phenyl-prop-yl]malonate.[Pubmed:26958401]
Acta Crystallogr E Crystallogr Commun. 2016 Jan 27;72(Pt 2):257-60.
In the racemic title compound, C24H25NO9, the dihedral angle between the planes of the two benzene-ring systems is 80.16 (6) degrees , while the side-chain conformation is stabilized by a methyl-ene-carboxyl C-Hcdots, three dots, centeredO hydrogen bond. Weak inter-molecular C-Hcdots, three dots, centeredO hydrogen bonds form inversion dimers [graph set R 2 (2)(16)] which are linked into chains extending along a. Further C-Hcdots, three dots, centeredO hydrogen bonding extends the structure along b through cyclic R 2 (2)(10) motifs. Although no pi-pi aromatic ring inter-actions are present in the structure, C-Hcdots, three dots, centeredpi ring inter-actions across c generate an overall three-dimensional supra-molecular structure.
Bi-directional ACET micropump for on-chip biological applications.[Pubmed:26790840]
Electrophoresis. 2016 Mar;37(5-6):719-26.
The ability to control and pump high ionic strength fluids inside microchannels forms a major advantage for clinical diagnostics and drug screening processes, where high conductive biological and physiological buffers are used. Despite the known potential of AC electro-thermal (ACET) effect in different biomedical applications, comparatively little is known about controlling the velocity and direction of fluid inside the chip. Here, we proposed to discretize the conventional electrodes to form various asymmetric electrode structures in order to control the fluid direction by simple switching the appropriate electric potential applied to the discretized electrodes. The ACET pumping effect was numerically studied by solving electrical, thermal and hydrodynamic multi-physic coupled equations to optimize the geometrical dimensions of the discretized system. PBS solutions with different ionic strength were seeded with 1 mum sized fluorescent particles and electrothermally driven fluid motion was observed inside the channel for different electrode structures. Experimental analyses confirm that the proposed micropump is efficient for a conductivity range between 0.1 and 1 S/m and the efficiency improves by increasing the voltage amplitude. Behavior of the proposed electrode-electrolyte system is discussed by lumped circuit model. Frequency response of system illustrated that the optimal frequency range increases by increasing the conductivity of medium. For 0.18 S/m PBS solution, the constant pumping effect was observed at frequency range between 100 kHz and 1 MHz, while frequency range of 100 kHz to 5 MHZ was observed for 0.42 S/m. The characteristics of experimental results were in good agreement with the theoretical model.
Crystal structure of (E)-4-(acet-oxy-imino)-N-allyl-3-isopropyl-2,6-di-phenyl-piperi-dine-1-carbo-thio -amide.[Pubmed:26396787]
Acta Crystallogr E Crystallogr Commun. 2015 Jul 4;71(Pt 8):o542-3.
The title compound, C26H31N3O2S, crystallizes with two mol-ecules (A and B) in the asymmetric unit. In each case, the piperidine ring exists in a twist-boat conformation. The dihedral angle between the phenyl rings is 46.16 (12) degrees in mol-ecule A and 44.95 (12) degrees in mol-ecule B. In both mol-ecules, the allyl side chain is disordered over two orientations in a 0.649 (9):0.351 (9) ratio for mol-ecule A and 0.826 (10):0.174 (10) ratio for mol-ecule B. In the crystal, neither mol-ecule forms a hydrogen bond from its N-H group, presumably due to steric hindrance. A+A and B+B inversion dimers are formed, linked by pairs of weak C-Hcdots, three dots, centeredO hydrogen bonds enclosing R 2 (2)(22) ring motifs.
ACET is a highly potent and specific kainate receptor antagonist: characterisation and effects on hippocampal mossy fibre function.[Pubmed:18789344]
Neuropharmacology. 2009 Jan;56(1):121-30.
Kainate receptors (KARs) are involved in both NMDA receptor-independent long-term potentiation (LTP) and synaptic facilitation at mossy fibre synapses in the CA3 region of the hippocampus. However, the identity of the KAR subtypes involved remains controversial. Here we used a highly potent and selective GluK1 (formerly GluR5) antagonist (ACET) to elucidate roles of GluK1-containing KARs in these synaptic processes. We confirmed that ACET is an extremely potent GluK1 antagonist, with a Kb value of 1.4+/-0.2 nM. In contrast, ACET was ineffective at GluK2 (formerly GluR6) receptors at all concentrations tested (up to 100 microM) and had no effect at GluK3 (formerly GluR7) when tested at 1 microM. The X-ray crystal structure of ACET bound to the ligand binding core of GluK1 was similar to the UBP310-GluK1 complex. In the CA1 region of hippocampal slices, ACET was effective at blocking the depression of both fEPSPs and monosynaptically evoked GABAergic transmission induced by ATPA, a GluK1 selective agonist. In the CA3 region of the hippocampus, ACET blocked the induction of NMDA receptor-independent mossy fibre LTP. To directly investigate the role of pre-synaptic GluK1-containing KARs we combined patch-clamp electrophysiology and 2-photon microscopy to image Ca2+ dynamics in individual giant mossy fibre boutons. ACET consistently reduced short-term facilitation of pre-synaptic calcium transients induced by 5 action potentials evoked at 20-25Hz. Taken together our data provide further evidence for a physiological role of GluK1-containing KARs in synaptic facilitation and LTP induction at mossy fibre-CA3 synapses.
Synthesis and pharmacological characterization of N3-substituted willardiine derivatives: role of the substituent at the 5-position of the uracil ring in the development of highly potent and selective GLUK5 kainate receptor antagonists.[Pubmed:17348638]
J Med Chem. 2007 Apr 5;50(7):1558-70.
Some N3-substituted analogues of willardiine such as 11 and 13 are selective kainate receptor antagonists. In an attempt to improve the potency and selectivity for kainate receptors, a range of analogues of 11 and 13 were synthesized with 5-substituents on the uracil ring. An X-ray crystal structure of the 5-methyl analogue of 13 bound to GLUK5 revealed that there was allowed volume around the 4- and 5-positions of the thiophene ring, and therefore the 4,5-dibromo and 5-phenyl (67) analogues were synthesized. Compound 67 (ACET) demonstrated low nanomolar antagonist potency on native and recombinant GLUK5-containing kainate receptors (KB values of 7 +/- 1 and 5 +/- 1 nM for antagonism of recombinant human GLUK5 and GLUK5/GLUK2, respectively) but displayed IC50 values >100 microM for antagonism of GLUA2, GLUK6, or GLUK6/GLUK2.


