A939572Stearoyl-CoA desaturase1 (SCD1) inhibitor CAS# 1032229-33-6 |
- PF-04620110
Catalog No.:BCC2335
CAS No.:1109276-89-2
- Tolcapone
Catalog No.:BCC2334
CAS No.:134308-13-7
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- Lonafarnib
Catalog No.:BCC2331
CAS No.:193275-84-2
- FK866 (APO866)
Catalog No.:BCC2332
CAS No.:658084-64-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1032229-33-6 | SDF | Download SDF |
| PubChem ID | 24905400 | Appearance | Powder |
| Formula | C20H22ClN3O3 | M.Wt | 387.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | stearoyl-CoA desaturase (SCD) inhibitor; SCD-inhibitor | ||
| Solubility | DMSO : 100 mg/mL (257.82 mM; Need ultrasonic) | ||
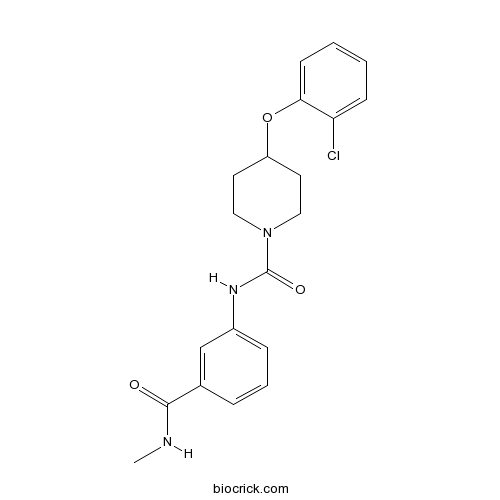
| Chemical Name | 4-(2-chlorophenoxy)-N-[3-(methylcarbamoyl)phenyl]piperidine-1-carboxamide | ||
| SMILES | CNC(=O)C1=CC(=CC=C1)NC(=O)N2CCC(CC2)OC3=CC=CC=C3Cl | ||
| Standard InChIKey | DPYTYQFYDLYWHZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H22ClN3O3/c1-22-19(25)14-5-4-6-15(13-14)23-20(26)24-11-9-16(10-12-24)27-18-8-3-2-7-17(18)21/h2-8,13,16H,9-12H2,1H3,(H,22,25)(H,23,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A939572 is a potent, and orally bioavailable SCD1 inhibitor with IC50 values of <4 nM and 37 nM for mSCD1 and hSCD1, respectively.In Vitro:A939572 exhibits robust in vivo activity with dose-dependent desaturation index lowering effects[1].
A939572 is a small molecule that specifically inhibits SCD1 enzymatic activity. A939572 demonstrates a significant dose-dependent decrease in proliferation in Caki1, A498, Caki2, and ACHN at day 5 (IC50s of 65 nM, 50 nM, 65 nM, and 6 nM, respectively). In A939572 (SCDi) treated Caki1 and A498 cells, all five ER stress related genes are expressed at significantly increased levels compared to DMSO+BSA control, and this elevated expression can be blocked with the addition of OA-BSA[2].In Vivo:Athymic nude (nu/nu) mice bearing A498 ccRCC xenografts are treated with A939572 (30mg/kg, p.o.) and Tem individually or in combination over the course of four weeks, and tumor volume (mm3) is recorded. A939572 and Tem monotherapy generate similar growth responses with approximately 20-30% reductions in tumor volume (vs. placebo control) being observed upon study completion, with values reaching statistical significance only within the last week of treatment. The combination group yields over a 60% decrease in tumor volume (vs. placebo control) by study completion with significant reductions recorded after approximately 1 week of treatment[2]. References: | |||||

A939572 Dilution Calculator

A939572 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5782 mL | 12.8912 mL | 25.7825 mL | 51.565 mL | 64.4562 mL |
| 5 mM | 0.5156 mL | 2.5782 mL | 5.1565 mL | 10.313 mL | 12.8912 mL |
| 10 mM | 0.2578 mL | 1.2891 mL | 2.5782 mL | 5.1565 mL | 6.4456 mL |
| 50 mM | 0.0516 mL | 0.2578 mL | 0.5156 mL | 1.0313 mL | 1.2891 mL |
| 100 mM | 0.0258 mL | 0.1289 mL | 0.2578 mL | 0.5156 mL | 0.6446 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A939572 is a potent and orally bioavailable inhibitor of stearoyl-CoA desaturase1 (SCD1) with IC50 value of 37nM [1].
SCD is a microsomal enzyme that catalyzes the biosynthesis of monounsaturated fatty acids. One member of SCD family, SCD1, is regulated by dietary and hormonal factors and is proved to play an important role in lipid metabolism and body weight control. Thus, SCD1 is a target for the treatment of obesity and diabetes. A939572 is a synthetic inhibitor of SCD1 with improved inhibitory activity and lipophilicity than its parent compound. It shows inhibition of mouse SCD1 and human SCD1 with IC50 values of <4nM and 37nM, respectively. In addition, A939572 has no inhibitory activity to the co-enzymes of SCD1, cytochrome b5 and cytochrome b5 reductase. It suggests that A939572 interacts with SCD1 directly and specifically. Furthermore, A939572 shows high oral bioavailability in mice [1].
References:
[1] Xin Z, Zhao H, Serby M D, et al. Discovery of piperidine-aryl urea-based stearoyl-CoA desaturase 1 inhibitors. Bioorganic & medicinal chemistry letters, 2008, 18(15): 4298-4302.
- Fmoc-Cys(Trt)-OH
Catalog No.:BCC3479
CAS No.:103213-32-7
- Fmoc-Tyr(3,5-I2)-OH
Catalog No.:BCC3264
CAS No.:103213-31-6
- Pranlukast
Catalog No.:BCC4827
CAS No.:103177-37-3
- 14-Norpseurotin A
Catalog No.:BCN7262
CAS No.:1031727-34-0
- UNC 3230
Catalog No.:BCC5618
CAS No.:1031602-63-7
- 4-(4-(Dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrile hydrobromide
Catalog No.:BCC8648
CAS No.:103146-26-5
- ABT-046
Catalog No.:BCC1326
CAS No.:1031336-60-3
- Kinetensin (human)
Catalog No.:BCC5845
CAS No.:103131-69-7
- 2-Amino-6-chloropurine
Catalog No.:BCC8540
CAS No.:10310-21-1
- Bakuchiol
Catalog No.:BCN5845
CAS No.:10309-37-2
- AS 2034178
Catalog No.:BCC7996
CAS No.:1030846-42-4
- MK-8245
Catalog No.:BCC2299
CAS No.:1030612-90-8
- D-Arabinose
Catalog No.:BCN3791
CAS No.:10323-20-3
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- L-655,240
Catalog No.:BCC7156
CAS No.:103253-15-2
- BAY 80-6946 (Copanlisib)
Catalog No.:BCC4986
CAS No.:1032568-63-0
- GNE-477
Catalog No.:BCC8049
CAS No.:1032754-81-6
- GDC-0980 (RG7422)
Catalog No.:BCC4992
CAS No.:1032754-93-0
- PTIQ
Catalog No.:BCC7953
CAS No.:1032822-42-6
- GSK1292263
Catalog No.:BCC3786
CAS No.:1032823-75-8
- LDK378
Catalog No.:BCC3691
CAS No.:1032900-25-6
- Taltirelin
Catalog No.:BCC5271
CAS No.:103300-74-9
- Pre-schisanartanin B
Catalog No.:BCN5846
CAS No.:1033288-92-4
- 3-Oxo-4-aza-5-alpha-androstane-17β-carboxylic acid
Catalog No.:BCC8641
CAS No.:103335-55-3
Inhibition of stearoyl CoA desaturase-1 activity suppresses tumour progression and improves prognosis in human bladder cancer.[Pubmed:30592142]
J Cell Mol Med. 2019 Mar;23(3):2064-2076.
Urinary bladder neoplasm is one of the most common cancers worldwide. Cancer stem cells (CSCs) have been proven to be an important cause of cancer progression and poor prognosis. In the present study, we established bladder CSCs and identified the crucial differentially expressed genes (DEGs) between these cells and parental bladder cancer cells. Analyses of bioinformatics data and clinical samples from local hospitals showed that stearoyl CoA desaturase-1 (SCD) was the key factor among the DEGs. A significant correlation between SCD gene expression and poor prognosis among patients with bladder cancer was observed in our data. Loss-of-function experiments further revealed that the SCD inhibitor A939572 and SCD gene interference reduced cell proliferation and invasion. The above data suggest that SCD may serve as a novel marker for the prediction of tumour progression and poor prognosis in patients with bladder cancer.
Stearoyl-CoA desaturase 1 deficiency reduces lipid accumulation in the heart by activating lipolysis independently of peroxisome proliferator-activated receptor alpha.[Pubmed:27751891]
Biochim Biophys Acta. 2016 Dec;1861(12 Pt A):2029-2037.
Stearoyl-CoA desaturase 1 (SCD1) has recently been shown to be a critical control point in the regulation of cardiac metabolism and function. Peroxisome proliferator-activated receptor alpha (PPARalpha) is an important regulator of myocardial fatty acid uptake and utilization. The present study used SCD1 and PPARalpha double knockout (SCD1(-/-)/PPARalpha(-/-)) mice to test the hypothesis that PPARalpha is involved in metabolic changes in the heart that are caused by SCD1 downregulation/inhibition. SCD1 deficiency decreased the intracellular content of free fatty acids, triglycerides, and ceramide in the heart of SCD1(-/-) and SCD1(-/-)/PPARalpha(-/-) mice. SCD1 ablation in PPARalpha(-/-) mice decreased diacylglycerol content in cardiomyocytes. These results indicate that the reduction of fat accumulation in the heart associated with SCD1 deficiency occurs independently of the PPARalpha pathway. To elucidate the mechanism of the observed changes, we treated HL-1 cardiomyocytes with the SCD1 inhibitor A939572 and/or PPARalpha inhibitor GW6471. SCD1 inhibition decreased the level of lipogenic proteins and increased lipolysis, reflected by a decrease in the content of adipose triglyceride lipase inhibitor G0S2 and a decrease in the ratio of phosphorylated hormone-sensitive lipase (HSL) at Ser565 to HSL (pHSL[Ser565]/HSL). PPARalpha inhibition alone did not affect the aforementioned protein levels. Finally, PPARalpha inhibition decreased the phosphorylation level of 5'-adenosine monophosphate-activated protein kinase, indicating lower mitochondrial fatty acid oxidation. In summary, SCD1 ablation/inhibition decreased cardiac lipid content independently of the action of PPARalpha by reducing lipogenesis and activating lipolysis. The present data suggest that SCD1 is an important component in maintaining proper cardiac lipid metabolism.
Monounsaturated fatty acids generated via stearoyl CoA desaturase-1 are endogenous inhibitors of fatty acid amide hydrolase.[Pubmed:24191036]
Proc Natl Acad Sci U S A. 2013 Nov 19;110(47):18832-7.
High-fat diet (HFD)-induced obesity and insulin resistance are associated with increased activity of the endocannabinoid/CB1 receptor (CB1R) system that promotes the hepatic expression of lipogenic genes, including stearoyl-CoA desaturase-1 (SCD1). Mice deficient in CB1R or SCD1 remain lean and insulin-sensitive on an HFD, suggesting a functional link between the two systems. The HFD-induced increase in the hepatic levels of the endocannabinoid anandamide [i.e., arachidonoylethanolamide (AEA)] has been attributed to reduced activity of the AEA-degrading enzyme fatty acid amide hydrolase (FAAH). Here we show that HFD-induced increased hepatic AEA levels and decreased FAAH activity are absent in SCD1(-/-) mice, and the monounsaturated fatty acid (MUFA) products of SCD1, palmitoleic and oleic acid, inhibit FAAH activity in vitro at low micromolar concentrations. HFD markedly increases hepatic SCD1 activity in WT mice as well as in CB1R(-/-) mice with transgenic reexpression of CB1R in hepatocytes, but not in global CB1R(-/-) mice. Treatment of HFD-fed mice with the SCD1 inhibitor A939572 prevents the diet-induced reduction of hepatic FAAH activity, normalizes hepatic AEA levels, and improves insulin sensitivity. SCD1(-/-) mice on an HFD remain insulin-sensitive, but develop glucose intolerance and insulin resistance in response to chronic treatment with the FAAH inhibitor URB597. An HFD rich in MUFA or feeding mice pure oleic acid fail to inhibit hepatic FAAH activity. We conclude that MUFAs generated via SCD1 activity, but not diet-derived MUFAs, function as endogenous FAAH inhibitors mediating the HFD-induced increase in hepatic AEA, which then activates hepatic CB1R to induce insulin resistance.
Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma.[Pubmed:23633458]
Clin Cancer Res. 2013 May 1;19(9):2368-80.
PURPOSE: We set out to identify Stearoyl-CoA desaturase 1 (SCD1) as a novel molecular target in clear cell renal cell carcinoma (ccRCC) and examine its role in tumor cell growth and viability in vitro and in vivo independently as well as in combination with current U.S. Food and Drug Administration (FDA)-approved regimens. EXPERIMENTAL DESIGN: Patient normal and ccRCC tissue samples and cell lines were examined for SCD1 expression. Genetic knockdown models and targeted inhibition of SCD1 through use of a small molecule inhibitor, A939572, were analyzed for growth, apoptosis, and alterations in gene expression using gene array analysis. Therapeutic models of synergy were evaluated utilizing pharmacologic inhibition of SCD1 with the tyrosine kinase inhibitors (TKI) sunitinib and pazopanib, and the mTOR inhibitor temsirolimus. RESULTS: Our studies identify increased SCD1 expression in all stages of ccRCC. Both genetic knockdown and pharmacologic inhibition of SCD1 decreased tumor cell proliferation and induced apoptosis in vitro and in vivo. Upon gene array, quantitative real-time PCR, and protein analysis of A939572-treated or SCD1 lentiviral knockdown samples, induction of endoplasmic reticulum stress response signaling was observed, providing mechanistic insight for SCD1 activity in ccRCC. Furthermore, combinatorial application of A939572 with temsirolimus synergistically inhibited tumor growth in vitro and in vivo. CONCLUSIONS: Increased SCD1 expression supports ccRCC viability and therefore we propose it as a novel molecular target for therapy either independently or in combination with an mTOR inhibitor for patients whose disease cannot be remedied with surgical intervention, such as in cases of advanced or metastatic disease.
Loss of stearoyl-CoA desaturase activity leads to free cholesterol synthesis through increased Xbp-1 splicing.[Pubmed:20923962]
Am J Physiol Endocrinol Metab. 2010 Dec;299(6):E1066-75.
Stearoyl-CoA desaturase-1 (SCD-1) is the rate-limiting enzyme in the biosynthesis of monounsaturated fatty acids (MUFA), which are required for efficient neutral lipid esterification. In the present investigation, we demonstrate that loss of SCD-1 activity increases free cholesterol (FC) content and induces Xbp-1 splicing. We assessed the small molecule SCD-1 inhibitor A939572 on [(14)C]stearate incorporation into neutral lipids and found its incorporation into triglyceride was unaffected, whereas labeled cholesteryl ester (CE) content was notably diminished. Using either A939572 or liver knockout mice (LKO), we show that loss of SCD-1 activity increases FC levels and activates the liver X receptor (LXR) pathway. Using adenoviral delivery of an active form of X-box binding protein-1 (Xbp-1; Xbp-1s), we show increased sterol synthesis only when cells lack the ability to generate MUFA. The results of the cell-based model were confirmed in LKO mice where fasting-refeeding decreased CE, increased FC, and increased Xbp-1s. On the basis of the present data, we conclude that SCD-1 activity is required for efficient cholesterol esterification to MUFA and that loss of its activity increases Xbp-1s-mediated FC synthesis. It is likely that the accumulation of FC enhances Xbp-1 splicing, induces LXR transcriptional activity, and increases ABCA1 (ATP-binding cassette transporter A1) expression to maintain cholesterol homeostasis.


