6-IodonordihydrocapsaicinVanilloid receptor antagonist CAS# 859171-97-4 |
- Edoxaban tosylate monohydrate
Catalog No.:BCC1545
CAS No.:1229194-11-9
- Otamixaban
Catalog No.:BCC1827
CAS No.:193153-04-7
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- Rivaroxaban
Catalog No.:BCC2292
CAS No.:366789-02-8
- Edoxaban
Catalog No.:BCC1543
CAS No.:480449-70-5
- Apixaban
Catalog No.:BCC2295
CAS No.:503612-47-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 859171-97-4 | SDF | Download SDF |
| PubChem ID | 5149140 | Appearance | Powder |
| Formula | C17H26INO3 | M.Wt | 419.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in ethanol and to 100 mM in DMSO | ||
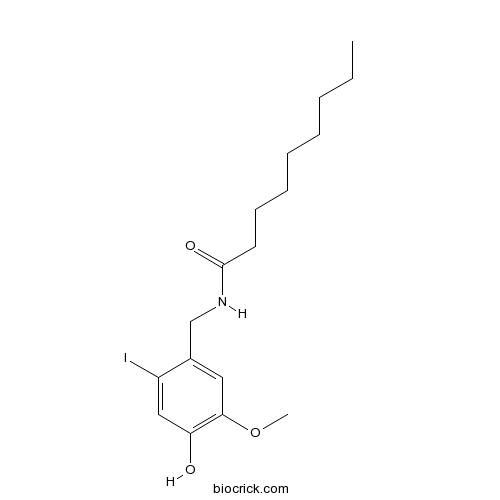
| Chemical Name | N-[(4-hydroxy-2-iodo-5-methoxyphenyl)methyl]nonanamide | ||
| SMILES | CCCCCCCCC(=O)NCC1=CC(=C(C=C1I)O)OC | ||
| Standard InChIKey | AAORACFZMYMFCG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H26INO3/c1-3-4-5-6-7-8-9-17(21)19-12-13-10-16(22-2)15(20)11-14(13)18/h10-11,20H,3-9,12H2,1-2H3,(H,19,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent competitive vanilloid TRPV1 (VR1) receptor antagonist (IC50 = 10 nM). Inhibits capsaicin-induced [Ca2+]i increase in rat DRG neurons, and guinea pig bladder and trachea contractions in vitro. |

6-Iodonordihydrocapsaicin Dilution Calculator

6-Iodonordihydrocapsaicin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3849 mL | 11.9246 mL | 23.8493 mL | 47.6985 mL | 59.6232 mL |
| 5 mM | 0.477 mL | 2.3849 mL | 4.7699 mL | 9.5397 mL | 11.9246 mL |
| 10 mM | 0.2385 mL | 1.1925 mL | 2.3849 mL | 4.7699 mL | 5.9623 mL |
| 50 mM | 0.0477 mL | 0.2385 mL | 0.477 mL | 0.954 mL | 1.1925 mL |
| 100 mM | 0.0238 mL | 0.1192 mL | 0.2385 mL | 0.477 mL | 0.5962 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 10 nm against 100 nm capsaicin
The vanilloid TRPV1 receptor, also known as VR1 receptor, belongs to the large family of ‘transient receptor potential’ (TRP). TRPV1 functions as a molecular integrator of nociceptive stimuli, including heat, protons and plant toxins, and is most abundant in peripheral sensory fibers of the C and Ad type. 6-iodo-nordihydrocapsaicin is a potent TRPV1 antagonist.
In vitro: Using human recombinant TRPV1, 6-Iodonordihydrocapsaicin (IC50=10 nm against 100 nm capsaicin) was about four times more potent than the prototypical TRPV1 antagonist, capsazepine [1].
In vivo: 6-Iodonordihydrocapsaicin was tested against capsaicin also on native TRPV1 in: (i) rat dorsal root ganglion neurons in culture; (ii) guinea-pig urinary bladder; and (iii) guinea-pig bronchi. In all cases, except for the guineapig bronchi, the compound was significantly more potent than capsazepine as a TRPV1 antagonist [1].
Clinical trial: Up to now, 6-Iodonordihydrocapsaicin is still in the preclinical development stage.
Reference:
[1] Appendino G, Harrison S, De Petrocellis L, Daddario N, Bianchi F, Schiano Moriello A, Trevisani M, Benvenuti F, Geppetti P, Di Marzo V. Halogenation of a capsaicin analogue leads to novel vanilloid TRPV1 receptor antagonists. Br J Pharmacol. 2003 Aug;139(8):1417-24.
- Lincomycin hydrochloride
Catalog No.:BCC9011
CAS No.:859-18-7
- prim-O-Glucosylangelicain
Catalog No.:BCN4409
CAS No.:85889-15-2
- 1,3-Dipropyl-8-phenylxanthine
Catalog No.:BCC6664
CAS No.:85872-53-3
- Marsdenoside F
Catalog No.:BCN4564
CAS No.:858360-61-9
- Vialinin A
Catalog No.:BCC2367
CAS No.:858134-23-3
- 3-O-Benzyl estrone
Catalog No.:BCC8638
CAS No.:858-98-0
- (-)-Quinpirole hydrochloride
Catalog No.:BCC6917
CAS No.:85798-08-9
- 11,12-Di-O-acetyltenacigenin B
Catalog No.:BCN4565
CAS No.:857897-01-9
- Motesanib Diphosphate (AMG-706)
Catalog No.:BCC2477
CAS No.:857876-30-3
- Polygalaxanthone XI
Catalog No.:BCN7366
CAS No.:857859-82-6
- Alstolenine
Catalog No.:BCN4808
CAS No.:85769-33-1
- Longistylumphylline A
Catalog No.:BCN4408
CAS No.:857672-34-5
- 4-O-Methylhonokiol
Catalog No.:BCN8474
CAS No.:68592-15-4
- Acetyl meldrum's acid
Catalog No.:BCC8805
CAS No.:85920-63-4
- AGN 205728
Catalog No.:BCC5418
CAS No.:859498-05-8
- GR 125487 sulfamate
Catalog No.:BCC7142
CAS No.:859502-43-5
- FIT
Catalog No.:BCC7082
CAS No.:85951-63-9
- BMS-690514
Catalog No.:BCC1430
CAS No.:859853-30-8
- Anemosapogenin
Catalog No.:BCN2454
CAS No.:85999-40-2
- Carbazole
Catalog No.:BCN6903
CAS No.:86-74-8
- Benzoyleneurea
Catalog No.:BCC8865
CAS No.:86-96-4
- Cyclovirobuxine
Catalog No.:BCN5965
CAS No.:860-79-7
- AZD7762
Catalog No.:BCC2555
CAS No.:860352-01-8
- Fmoc-Cys(Acm)-OH
Catalog No.:BCC3473
CAS No.:86060-81-3
Blockade of Cannabinoid CB1 Receptors in the Dorsal Periaqueductal Gray Unmasks the Antinociceptive Effect of Local Injections of Anandamide in Mice.[Pubmed:29046638]
Front Pharmacol. 2017 Oct 4;8:695.
Divergent results in pain management account for the growing number of studies aiming at elucidating the pharmacology of the endocannabinoid/endovanilloid anandamide (AEA) within several pain-related brain structures. For instance, the stimulation of both Transient Receptor Potential Vanilloid type 1 (TRPV1) and Cannabinoid type 1 (CB1) receptors led to paradoxical effects on nociception. Here, we attempted to propose a clear and reproducible methodology to achieve the antinociceptive effect of exogenous AEA within the dorsal periaqueductal gray (dPAG) of mice exposed to the tail-flick test. Accordingly, male Swiss mice received intra-dPAG injection of AEA (CB1/TRPV1 agonist), capsaicin (TRPV1 agonist), WIN (CB1 agonist), AM251 (CB1 antagonist), and 6-Iodonordihydrocapsaicin (6-IODO) (TRPV1 selective antagonist) and their nociceptive response was assessed with the tail-flick test. In order to assess AEA effects on nociception specifically at vanilloid or cannabinoid (CB) substrates into the dPAG, mice underwent an intrinsically inactive dose of AM251 or 6-IODO followed by local AEA injections and were subjected to the same test. While intra-dPAG AEA did not change acute pain, local injections of capsaicin or WIN induced a marked TRPV1- and CB1-dependent antinociceptive effect, respectively. Regarding the role of AEA specifically at CB/vanilloid substrates, while the blockade of TRPV1 did not change the lack of effects of intra-dPAG AEA on nociception, local pre-treatment of AM251, a CB1 antagonist, led to a clear AEA-induced antinociception. It seems that the exogenous AEA-induced antinociception is unmasked when it selectively binds to vanilloid substrates, which might be useful to address acute pain in basic and perhaps clinical trials.
The TRPV1 channel in rodents is a major target for antinociceptive effect of the probiotic Lactobacillus reuteri DSM 17938.[Pubmed:26084409]
J Physiol. 2015 Sep 1;593(17):3943-57.
Certain probiotic bacteria have been shown to reduce distension-dependent gut pain, but the mechanisms involved remain obscure. Live luminal Lactobacillus reuteri (DSM 17938) and its conditioned medium dose dependently reduced jejunal spinal nerve firing evoked by distension or capsaicin, and 80% of this response was blocked by a specific TRPV1 channel antagonist or in TRPV1 knockout mice. The specificity of DSM action on TRPV1 was further confirmed by its inhibition of capsaicin-induced intracellular calcium increases in dorsal root ganglion neurons. Another lactobacillus with ability to reduce gut pain did not modify this response. Prior feeding of rats with DSM inhibited the bradycardia induced by painful gastric distension. These results offer a system for the screening of new and improved candidate bacteria that may be useful as novel therapeutic adjuncts in gut pain. Certain bacteria exert visceral antinociceptive activity, but the mechanisms involved are not determined. Lactobacillus reuteri DSM 17938 was examined since it may be antinociceptive in children. Since transient receptor potential vanilloid 1 (TRPV1) channel activity may mediate nociceptive signals, we hypothesized that TRPV1 current is inhibited by DSM. We tested this by examining the effect of DSM on the firing frequency of spinal nerve fibres in murine jejunal mesenteric nerve bundles following serosal application of capsaicin. We also measured the effects of DSM on capsaicin-evoked increase in intracellular Ca(2+) or ionic current in dorsal root ganglion (DRG) neurons. Furthermore, we tested the in vivo antinociceptive effects of oral DSM on gastric distension in rats. Live DSM reduced the response of capsaicin- and distension-evoked firing of spinal nerve action potentials (238 +/- 27.5% vs. 129 +/- 17%). DSM also reduced the capsaicin-evoked TRPV1 ionic current in DRG neuronal primary culture from 83 +/- 11% to 41 +/- 8% of the initial response to capsaicin only. Another lactobacillus (Lactobacillus rhamnosus JB-1) with known visceral anti-nociceptive activity did not have these effects. DSM also inhibited capsaicin-evoked Ca(2+) increase in DRG neurons; an increase in Ca(2+) fluorescence intensity ratio of 2.36 +/- 0.31 evoked by capsaicin was reduced to 1.25 +/- 0.04. DSM releasable products (conditioned medium) mimicked DSM inhibition of capsaicin-evoked excitability. The TRPV1 antagonist 6-Iodonordihydrocapsaicin or the use of TRPV1 knock-out mice revealed that TRPV1 channels mediate about 80% of the inhibitory effect of DSM on mesenteric nerve response to high intensity gut distension. Finally, feeding with DSM inhibited perception in rats of painful gastric distension. Our results identify a specific target channel for a probiotic with potential therapeutic properties.
Anxiogenic-like effects induced by hemopressin in rats.[Pubmed:25462856]
Pharmacol Biochem Behav. 2015 Feb;129:7-13.
Hemopressin (PVNFKFLSH; HP) is an orally active peptide derived from rat hemoglobin alpha-chain that could act as an inverse agonist at cannabinoid type 1 receptors (CB1). Here, we aim to investigate possible behavioral effects of HP in male Wistar rats tested in the elevated plus maze (EPM), following HP intraperitoneal (i.p., 0.05 mg/kg), oral (P.O., 0.05 and 0.5 mg/kg) or intracerebroventricular (I.C.V., 3 and 10 nmol) administration. HP induced a decrease in EPM open arm exploration, indicating an anxiogenic-like effect. However, i.p. administration of HP (1 mg/kg) followed by mass spectrometry analysis of brain-peptide extracts suggested that the intact HP does not cross the blood brain barrier. I.C.V. administrated HP produced anxiogenic-like effects that were prevented by Transient Receptor Potential Vanilloid Type 1 (TRPV1) antagonists, 6-Iodonordihydrocapsaicin (1 nmol) or SB366791 (1 nmol), but not by the CB1 receptor antagonist AM251 (0.1 and 1 nmol). Altogether, these data suggest that I.C.V. administrated HP induces anxiogenic-like effects by activating TRPV1 receptors. The similar anxiogenic effects observed after i.p. or P.O. administration could be due to HP fragment(s) crossing the blood brain barrier. The present results advance our knowledge about HP pharmacology and suggest concerns in future clinical studies.
An exploration of the estrogen receptor transcription activity of capsaicin analogues via an integrated approach based on in silico prediction and in vitro assays.[Pubmed:24747365]
Toxicol Lett. 2014 Jun 16;227(3):179-88.
Capsaicin has been considered as an alternative template of dichlorodiphenyl trichloroethane (DDT) in antifouling paint. However, information regarding the estrogenic activity of capsaicin analogues is rather limited in comparison to that of DDT analogues and their metabolites. We here explore the ER transcription activity of selected capsaicin analogues via an integrated approach based on in silico prediction and in vitro assays. Molecular simulation and the agonist/antagonist differential-docking screening identified 6-Iodonordihydrocapsaicin (6-I-CPS) as a weak ERalpha agonist, while anti-estrogenicity was expected for N-arachidonoyldopamine, capsazepine, dihydrocapsaicin, trichostatin A, and capsaicin. On the contrary, the large volume of analogues, such as phorbol 12-phenylacetate 13-acetate 20-homovanillate and phorbol 12,13-dinonanoate 20-homovanillate, cannot fit well with the ER cavity. The result of MVLN assay was in accord with the in silico prediction. 6-I-CPS was demonstrated to induce luciferase gene expression, while the other analogues of relatively small molecular volume reduced luciferase gene expression in MVLN cells, both in the absence and presence of estradiol. This finding suggested that the ER transcription activity of capsaicin analogues is generated at least partly through the ERalpha-mediated pathway. Moreover, receptor polymorphism analysis indicated that capsaicin analogues may exhibit diverse species selectivity for human beings and marine species.
Medial prefrontal cortex Transient Receptor Potential Vanilloid Type 1 (TRPV1) in the expression of contextual fear conditioning in Wistar rats.[Pubmed:23922023]
Psychopharmacology (Berl). 2014 Jan;231(1):149-57.
RATIONALE: Contextual fear is evoked by re-exposing an animal to an environment that has been previously paired with an aversive or unpleasant stimulus. It can be assessed by freezing and cardiovascular changes such as increase in mean arterial pressure and heart rate. A marked increase in neuronal activity is associated with contextual fear conditioning, especially in limbic structures involved with defense reactions, such as the ventral portion of medial prefrontal cortex. OBJECTIVE: Given the fact that transient receptor potential vanilloid type 1 (TRPV1) receptors could be involved in the expression of defensive behavior, the present work tested the hypothesis that TRPV1 manipulation in the ventromedial prefrontal cortex (vMPFC) modulates the expression of contextual conditioned fear. METHODS: Male Wistar rats received bilateral microinjections into the vMPFC of the TRPV1 receptor antagonists capsazepine (1, 10, and 60 nmol/200 nL) or 6-Iodonordihydrocapsaicin (3 nmol/200 nL), and the TRPV1 agonist capsaicin (1 nmol/200 nL) preceded by vehicle or 6-Iodonordihydrocapsaicin before re-exposure to the experimental chamber for 10 min, 48 h after conditioning in two different protocols distinct by their aversiveness. RESULTS: Both antagonists reduced the freezing and cardiovascular responses in the high aversive protocol. Capsaicin caused an increase in fear-associated responses that could be blocked by 6-Iodonordihydrocapsaicin. CONCLUSIONS: Our results indicate that TRPV1 receptors located in the vMPFC have a tonic involvement in the modulation of the expression of contextual fear conditioning.
The endocannabinoid and endovanilloid systems interact in the rat prelimbic medial prefrontal cortex to control anxiety-like behavior.[Pubmed:22691536]
Neuropharmacology. 2012 Aug;63(2):202-10.
Cannabinoid receptor 1 (CB(1)) agonists usually induce dose-dependent biphasic effects on anxiety-related responses. Low doses induce anxiolytic-like effects, whereas high doses are ineffective or anxiogenic, probably due to activation of Transient Receptor Potential Vanilloid Type 1 (TRPV(1)) channels. In this study we have investigated this hypothesis by verifying the effects of the CB(1)/TRPV(1) agonist ACEA injected into the prelimbic medial prefrontal cortex (PL) and the participation of endocannabinoids in the anxiolytic-like responses induced by TRPV(1) antagonism, using the elevated plus-maze (EPM) and the Vogel conflict test (VCT). Moreover, we verified the expression of these receptors in the PL by double labeling immunofluorescence. ACEA induced anxiolytic-like effect in the intermediate dose, which was attenuated by previous injection of AM251, a CB(1) receptor antagonist. The higher and ineffective ACEA dose caused anxiogenic- and anxiolytic-like effects, when injected after AM251 or the TRPV(1) antagonist 6-Iodonordihydrocapsaicin (6-I-CPS), respectively. Higher dose of 6-I-CPS induced anxiolytic-like effects both in the EPM and the VCT, which were prevented by previous administration of AM251. In addition, immunofluorescence showed that CB(1) and TRPV(1) receptors are closely located in the PL. These results indicate that the endocannabinoid and endovanilloid systems interact in the PL to control anxiety-like behavior.
The endocannabinoid N-arachidonoyldopamine (NADA) exerts neuroprotective effects after excitotoxic neuronal damage via cannabinoid receptor 1 (CB(1)).[Pubmed:22186081]
Neuropharmacology. 2012 Mar;62(4):1797-807.
Endocannabinoids exert numerous effects in the CNS under physiological and pathological conditions. The aim of the present study was to examine whether the endocannabinoid N-arachidonoyldopamine (NADA) may protect neurons in excitotoxically lesioned organotypic hippocampal slice cultures (OHSC). OHSC were excitotoxically lesioned by application of N-methyl-d-aspartate (NMDA, 50 muM) for 4 h and subsequently treated with different NADA concentrations (0.1 pM-50 muM) alone or in combination with cannabinoid receptor antagonists. NADA protected dentate gyrus granule cells and caused a slight reduction in the number of microglial cells. The number of degenerated neurons significantly decreased between 100 pM and 10 muM NADA (p < 0.05). To identify the responsive receptor type of NADA mediated neuroprotection, we applied the cannabinoid (CB) receptor 1 (CB(1)) inverse agonist/antagonist AM251, CB(2) inverse agonist/antagonist AM630, abnormal-cannabidiol (abn-CBD)-sensitive receptor antagonist O-1918, transient receptor potential channel V1 (TRPV1) antagonist 6-Iodonordihydrocapsaicin and A1 (TRPA1) antagonist HC-030031. Neuroprotective properties of low (1 nM) but not high (10 muM) NADA concentrations were solely blocked by AM251 and were absent in CB(1)(-/-) mice. AM630, O-1918, 6-Iodonordihydrocapsaicin and HC-030031 showed no effects at all NADA concentrations applied. Our findings demonstrate that NADA protects dentate gyrus granule cells by acting via CB(1). NADA reduced the number of microglial cells at distinct concentrations. TRPV1 and TRPA1 were not involved in NADA mediated neuroprotection. Thus, our data implicate that NADA mediated activation of neuronal CB(1) may serve as a novel pharmacological target to mitigate symptoms of neuronal damage.
Halogenation of a capsaicin analogue leads to novel vanilloid TRPV1 receptor antagonists.[Pubmed:12922928]
Br J Pharmacol. 2003 Aug;139(8):1417-24.
1. The C-5 halogenation of the vanillyl moiety of resiniferatoxin, an ultrapotent agonist of vanilloid TRPV1 receptors, results in a potent antagonist for these receptors. Here, we have synthesized a series of halogenated derivatives of 'synthetic capsaicin' (nonanoyl vanillamide=nordihydrocapsaicin) differing for the nature (iodine, bromine-chlorine) and the regiochemistry (C-5, C-6) of the halogenation. 2. The activity of these compounds was investigated on recombinant human TRPV1 receptors overexpressed in HEK-293 cells. None of the six compounds exerted any significant agonist activity, as assessed by measuring their effect on TRPV1-mediated calcium mobilization. Instead, all compounds antagonized, to various extents, the effect of capsaicin in this assay. 3. All 6-halo-nordihydrocapsaicins behaved as competitive antagonists against human TRPV1 according to the corresponding Schild's plots, and were more potent than the corresponding 5-halogenated analogues. The iodo-derivatives were more potent than the bromo- and chloro-derivatives. 4. Using human recombinant TRPV1, 6-iodo-nordihydrocapsaicin (IC(50)=10 nM against 100 nM capsaicin) was about four times more potent than the prototypical TRPV1 antagonist, capsazepine, and was tested against capsaicin also on native TRPV1 in: (i) rat dorsal root ganglion neurons in culture; (ii) guinea-pig urinary bladder; and (iii) guinea-pig bronchi. In all cases, except for the guinea-pig bronchi, the compound was significantly more potent than capsazepine as a TRPV1 antagonist. 5. In conclusion, 6-iodo-nordihydrocapsaicin, a stable and easily prepared compound, is a potent TRPV1 antagonist and a convenient replacement for capsazepine in most of the in vitro preparations currently used to assess the activity of putative vanilloid receptor agonists.


