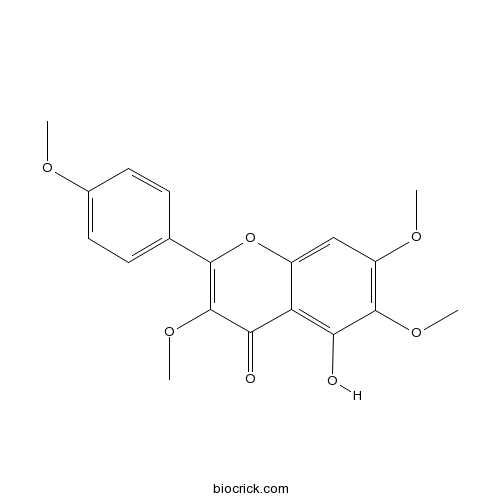
5-Hydroxy-3,6,7,4'-tetramethoxyflavoneCAS# 14787-34-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14787-34-9 | SDF | Download SDF |
| PubChem ID | 5318355 | Appearance | Yellow powder |
| Formula | C19H18O7 | M.Wt | 358.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-3,6,7-trimethoxy-2-(4-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2=C(C(=O)C3=C(C(=C(C=C3O2)OC)OC)O)OC | ||
| Standard InChIKey | ADNCDMHZHONBRR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18O7/c1-22-11-7-5-10(6-8-11)17-19(25-4)16(21)14-12(26-17)9-13(23-2)18(24-3)15(14)20/h5-9,20H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5-Hydroxy-3,6,7,4'-tetramethoxyflavone Dilution Calculator

5-Hydroxy-3,6,7,4'-tetramethoxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.791 mL | 13.9548 mL | 27.9096 mL | 55.8191 mL | 69.7739 mL |
| 5 mM | 0.5582 mL | 2.791 mL | 5.5819 mL | 11.1638 mL | 13.9548 mL |
| 10 mM | 0.2791 mL | 1.3955 mL | 2.791 mL | 5.5819 mL | 6.9774 mL |
| 50 mM | 0.0558 mL | 0.2791 mL | 0.5582 mL | 1.1164 mL | 1.3955 mL |
| 100 mM | 0.0279 mL | 0.1395 mL | 0.2791 mL | 0.5582 mL | 0.6977 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chrysosplenol C
Catalog No.:BCN9492
CAS No.:23370-16-3
- 2,4,3',4',6'-Penta-O-(3-methylbutanoyl)sucrose
Catalog No.:BCN9491
CAS No.:150302-84-4
- (-)-Sesamin 2,2'-diol
Catalog No.:BCN9490
CAS No.:1152441-87-6
- Vesticarpan
Catalog No.:BCN9489
CAS No.:69853-46-9
- 24,24-Dimethyl-5α-lanosta-9(11),25-dien-3β-ol
Catalog No.:BCN9488
CAS No.:33762-29-7
- 3-O-Debenzoylzeylenone
Catalog No.:BCN9487
CAS No.:1800008-77-8
- Methoxyimbricatin
Catalog No.:BCN9486
CAS No.:233759-30-3
- Flaccidin
Catalog No.:BCN9485
CAS No.:115531-76-5
- Rhodomyrtone
Catalog No.:BCN9484
CAS No.:468757-69-9
- Leptostachyol acetate
Catalog No.:BCN9483
CAS No.:35770-58-2
- 6-(3-Chloro-2-hydroxy-3-methylbutyl)-5,7-dimethoxycoumarin
Catalog No.:BCN9482
CAS No.:15575-50-5
- 2-Hydroxy-1,8-dimethoxyxanthone
Catalog No.:BCN9481
CAS No.:38974-76-4
- Phrymarolin V
Catalog No.:BCN9494
CAS No.:1449376-84-4
- Callosin
Catalog No.:BCN9495
CAS No.:166197-43-9
- Tetrahydroxymethoxychalcone
Catalog No.:BCN9496
CAS No.:197227-39-7
- Demethoxymatteucinol
Catalog No.:BCN9497
CAS No.:56297-79-1
- Glycocitrine I
Catalog No.:BCN9498
CAS No.:82354-36-7
- N-Methylatalaphylline
Catalog No.:BCN9499
CAS No.:28233-34-3
- Swietenidine B
Catalog No.:BCN9500
CAS No.:2721-56-4
- Trigothysoid P
Catalog No.:BCN9501
CAS No.:1501943-10-7
- Koenimbine
Catalog No.:BCN9502
CAS No.:21087-98-9
- 3,4,3'-Tri-O-methylflavellagic acid
Catalog No.:BCN9503
CAS No.:13756-49-5
- 3,2'-Dihydroxy-4,5-dimethoxybibenzyl
Catalog No.:BCN9504
CAS No.:212116-72-8
- Isomurrayafoline B
Catalog No.:BCN9505
CAS No.:107903-15-1
Compounds from the Fruits of the Popular European Medicinal Plant Vitex agnus-castus in Chemoprevention via NADP(H):Quinone Oxidoreductase Type 1 Induction.[Pubmed:23662135]
Evid Based Complement Alternat Med. 2013;2013:432829.
As part of our continuing efforts in the search for potential biologically active compounds from medicinal plants, we have isolated 18 compounds including two novel nitrogen containing diterpenes from extracts of the fruits of Vitex agnus-castus. These isolates, along with our previously obtained novel compound vitexlactam A (1), were evaluated for potential biological effects, including cancer chemoprevention. Chemically, the nitrogenous isolates were found to be two labdane diterpene alkaloids, each containing an alpha , beta -unsaturated gamma -lactam moiety. Structurally, they were elucidated to be 9 alpha -hydroxy-13(14)-labden-16,15-amide (2) and 6 beta -acetoxy-9 alpha -hydroxy-13(14)-labden-15,16-amide (3), which were named vitexlactams B and C, respectively. The 15 known isolates were identified as vitexilactone (4), rotundifuran (5), 8-epi-manoyl oxide (6), vitetrifolin D (7), spathulenol (8), cis-dihydro-dehydro-diconiferylalcohol-9-O- beta -D-glucoside (9), luteolin-7-O-glucoside (10), 5-hydroxy-3,6,7,4'-tetramethoxyflavone (11), casticin (12), artemetin (13), aucubin (14), agnuside (15), beta -sitosterol (16), p-hydroxybenzoic acid (17), and p-hydroxybenzoic acid glucose ester (18). All compound structures were determined/identified on the basis of 1D and/or 2D NMR and mass spectrometry techniques. Compounds 6, 8, 9, and 18 were reported from a Vitex spieces for the first time. The cancer chemopreventive potentials of these isolates were evaluated for NADP(H):quinone oxidoreductase type 1 (QR1) induction activity. Compound 7 demonstrated promising QR1 induction effect, while the new compound vitexlactam (3) was only slightly active.
Bioactive diterpenes from Callicarpa longissima.[Pubmed:22429052]
J Nat Prod. 2012 Apr 27;75(4):689-93.
Investigation of the leaves and twigs of Callicarpa longissima resulted in the isolation of four new compounds (1-4), callilongisins A-D, and five known compounds, ursolic acid, 3-oxoanticopalic acid, (E)-6beta-hydroxylabda-8(17),13-dien-15-oic acid, 5-hydroxy-3,6,7,4'-tetramethoxyflavone, and artemetin. Compounds 1-3 are 3,4-seco-abietane-type diterpenoids, and compound 4 is an analogue of a labdenoic-type diterpene. The structure of compound 1 was confirmed by X-ray crystallographic analysis. Cytotoxicity against a human prostate cancer cell line (PC3) and anti-inflammatory activities of the isolated compounds were evaluated.
Spasmolytic effects of Scrophularia nodosa extract on isolated rabbit intestine.[Pubmed:22186340]
Pak J Pharm Sci. 2012 Jan;25(1):267-75.
Scrophularia nodosa (figwort), an indigenous medicinal plant grows in moist and cultivated waste ground. It contains saponins, cardioactive glycosides, flavonoids, resin, sugar and organic acids. It is traditionally used for anti-inflammatory purpose and in skin disorders. It has diuretic and cardiac stimulant properties. The present studies were carried out on crude extract of Scrophularia nodosa and its n-hexane, chloroform, ethyl acetate, n-butanol and aqueous fractions. During phytochemical studies seven known compounds of flavonoid nature were isolated from the chloroform fraction of crude extract of S. nodosa. The structures of these compounds were elucidated by spectroscopic (UV, IR, Mass (EIMS, HREIMS) and NMR ((1)H-NMR, (13)C-NMR, DEPT, and (1)H-(1)H, COSY, HMQC, HMBC and NOESY) techniques. Compound 1 was identified as 5, 4`-hydroxy-3, 6, 7-trimethoxyflavone, compound 2 as 5-hydroxy-3,6,7,4'-tetramethoxyflavone, compound 3 as Centaurein, compound 4 as 5-hydroxy-7,8,2',3',4'-pentamethoxyflavone (Serpyllin), compound 5 as Kaempferol 7-O-alpha-L-rhamnopyranoside, compound 6 as sakuranetin 4'-O (6''-O-alpha-L-rhamnopyranosyl)-beta-D-glucopyranoside (Vitexoside) and compound 7 as Spinoside. Crude extract and its fractions were tested on isolated rabbit intestine (in vitro) for their effects. The results of crude extract and its fractions in different doses showed the decrease in normal movement of the smooth muscles of rabbit intestine (jejunum). The chloroform fraction showed maximum relaxant effect (77.37%) at 15mg/ml dose and aqueous fraction showed 38.56% spasmogenic response which was not present in the crude extract. Further study was carried out on different fractions to investigate the possible mechanism of action of S. nodosa extract. For this purpose spasmolytic effect of different fractions were compared with agonist and antagonist activities of standard drugs including adrenaline, atropine andacetylcholine (1x10(-2), 1x10(-4) and 10(-6) M conc.). It is concluded that the chemical constituents present in S. nodosa having spasmolytic action are possibly acting through muscarinic receptors.


