5-CarboxystrictosidineCAS# 34371-47-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 34371-47-6 | SDF | Download SDF |
| PubChem ID | 53463172 | Appearance | Powder |
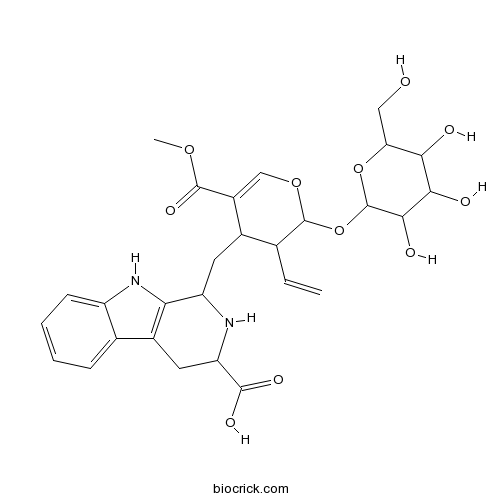
| Formula | C28H34N2O11 | M.Wt | 574.6 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-[[3-ethenyl-5-methoxycarbonyl-2-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,4-dihydro-2H-pyran-4-yl]methyl]-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid | ||
| SMILES | COC(=O)C1=COC(C(C1CC2C3=C(CC(N2)C(=O)O)C4=CC=CC=C4N3)C=C)OC5C(C(C(C(O5)CO)O)O)O | ||
| Standard InChIKey | LHKZIVMTXZLOTP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H34N2O11/c1-3-12-14(8-18-21-15(9-19(29-18)25(35)36)13-6-4-5-7-17(13)30-21)16(26(37)38-2)11-39-27(12)41-28-24(34)23(33)22(32)20(10-31)40-28/h3-7,11-12,14,18-20,22-24,27-34H,1,8-10H2,2H3,(H,35,36) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5-Carboxystrictosidine Dilution Calculator

5-Carboxystrictosidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7403 mL | 8.7017 mL | 17.4034 mL | 34.8068 mL | 43.5085 mL |
| 5 mM | 0.3481 mL | 1.7403 mL | 3.4807 mL | 6.9614 mL | 8.7017 mL |
| 10 mM | 0.174 mL | 0.8702 mL | 1.7403 mL | 3.4807 mL | 4.3509 mL |
| 50 mM | 0.0348 mL | 0.174 mL | 0.3481 mL | 0.6961 mL | 0.8702 mL |
| 100 mM | 0.0174 mL | 0.087 mL | 0.174 mL | 0.3481 mL | 0.4351 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,7-Dihydroxyxanthone
Catalog No.:BCN9407
CAS No.:64632-72-0
- Mumeose K
Catalog No.:BCN9406
CAS No.:2132384-01-9
- Tyramine hydrochloride
Catalog No.:BCN9405
CAS No.:60-19-5
- Symphonone I
Catalog No.:BCN9404
CAS No.:1235774-18-1
- Platachromone A
Catalog No.:BCN9403
CAS No.:1606149-62-5
- Methoxyeugenol
Catalog No.:BCN9402
CAS No.:6627-88-9
- 3β-Isodihydrocadambine
Catalog No.:BCN9401
CAS No.:62014-69-1
- p-Hydroxybenzaldehyde glucoside
Catalog No.:BCN9400
CAS No.:26993-16-8
- Batatasin I
Catalog No.:BCN9399
CAS No.:51415-00-0
- 10-Dehydrogingerdione
Catalog No.:BCN9398
CAS No.:99742-04-8
- Apocynoside I
Catalog No.:BCN9397
CAS No.:358721-31-0
- Methoxyadiantifoline
Catalog No.:BCN9396
CAS No.:115452-09-0
- Hyperxanthone
Catalog No.:BCN9409
CAS No.:99481-41-1
- Physaminimin D
Catalog No.:BCN9410
CAS No.:1582259-05-9
- Physaminimin C
Catalog No.:BCN9411
CAS No.:1582259-03-7
- Methyl (9Z,11E)-13-hydroxyoctadeca-9,11-dienoate
Catalog No.:BCN9412
CAS No.:109837-85-6
- Withaphysalin S
Catalog No.:BCN9413
CAS No.:949172-13-8
- Withaphysalin E
Catalog No.:BCN9414
CAS No.:118985-24-3
- Withaphysalin A
Catalog No.:BCN9415
CAS No.:57423-72-0
- Minisecolide C
Catalog No.:BCN9416
CAS No.:1967030-77-8
- 2,6-Dihydroxyxanthone
Catalog No.:BCN9417
CAS No.:838-11-9
- Dihydromikanolide
Catalog No.:BCN9418
CAS No.:23758-04-5
- 4,5-Dihydropiperlonguminine
Catalog No.:BCN9419
CAS No.:23512-53-0
- Deoxymikanolide
Catalog No.:BCN9420
CAS No.:23753-57-3
Molecular networking-based dereplication of strictosidine-derived monoterpene indole alkaloids from the curare ingredient Strychnos peckii.[Pubmed:31783430]
Rapid Commun Mass Spectrom. 2019 Nov 29:e8683.
RATIONALE: Monoterpene indole alkaloids (MIAs) are a large group of biologically active compounds produced by hundreds of plant species in numerous plant families, such as Apocynaceae, Loganiaceae and Rubiaceae. Although this diversity is biosynthetically intermediated by strictosidine, there are no works focused on the fragmentation patterns under collision-induced dissociation of strictosidine-derived alkaloids. METHODS: Initially, the alkaloid fingerprint of Strychnos peckii was established using leaf spray with tandem mass spectrometry (LS-MS/MS). Then, high-performance liquid chromatography coupled to tandem mass spectrometry (HPLC/MS/MS) analyses were carried out to focus on the patterns of neutral losses in product ion scan experiments with the leaf aqueous extract. Finally, the product ion spectra from a set of presumable strictosidine-type derivatives were analyzed and organized via molecular networking (MN), and dereplicated by manual interpretation of MS/MS spectra. RESULTS: LS-MS/MS allowed the tentative identification of strictosidine-derived alkaloids in the leaves of S. peckii, showing useful neutral losses for the dereplication of strictosidine analogues by HPLC/MS/MS experiments. The use of MN combined with manual interpretation of the fragmentation patterns highlighted characteristic fragmentation pathways, and allowed the tentative identification of strictosidine, desoxycordifoline, strictosidinic acid, 10-hydroxystrictosidine, 5-Carboxystrictosidine, lyaloside, 3,4-dehydrostrictosidine and strictosidine lactam. CONCLUSIONS: The use of MN combined with the analysis of the fragmentation patterns proved to be a useful strategy for the dereplication of strictosidine-derived MIAs from S. peckii, highlighting known and unprecedented structures, as well as useful diagnostic product ions. Therefore, this workflow is an effective approach for the characterization of strictosidine-type alkaloids in future dereplication works.
Bioprospection for antiplasmodial activity, and identification of bioactive metabolites of native plants species from the Mata Atlantica biome, Brazil.[Pubmed:31232104]
Nat Prod Res. 2019 Jun 24:1-6.
A total of 33 extracts of eleven different plants species from Mata Atlantica biome, Brazil, and different fractions of the bioactive extracts were evaluated against chloroquine-resistant Plasmodium falciparum W2 strain by PfLDH method and cytotoxicity to HepG2 cells by the MTT assay, and chemically characterized by LC-DAD-ESI-MS/MS analysis. The results allowed the identification of Alchornea glandulosa, Miconia latecrenata, and Psychotria suterella as the most active plant species. Different flavonoids and tannins in Alchornea glandulosa and Miconia latecrenata besides alkaloids in Psychotria suterella were identified. Bioguided fractionation of A. glandulosa and M. latecrenata leaves extracts led to fractions exhibiting high parasite growth inhibition. Seven known alkaloids were identified in the P. suterella extract, and of these, only 5-Carboxystrictosidine had been assayed for antiplasmodial activity what points to this species as the most promising among the eleven one assayed.
Total Syntheses of (-)-Secologanin, (-)-5-Carboxystrictosidine, and (-)-Rubenine.[Pubmed:31069870]
Chemistry. 2019 Jul 5;25(38):8996-9000.
The first enantioselective total syntheses of (-)-secologanin (1), (-)-5-Carboxystrictosidine (2), and (-)-rubenine (3) were accomplished in 10, 9, and 14 steps, respectively. The key transformation in the synthesis of 1 was a sequential anti-selective organocatalytic Michael reaction/Fukuyama reduction/spontaneous cyclization to form an optically active dihydropyran ring. In addition, the secologanin tetraacetate (16), which is a potential key intermediate for the bioinspired divergent syntheses of monoterpenoid indole alkaloids, was prepared in gram-scale in seven steps. The total syntheses of 2 and 3, which are classified as glycosylated monoterpenoid indole alkaloids, were achieved through bioinspired transformations such as a diastereoselective Pictet-Spengler reaction, a site- and stereoselective epoxidation, and a site-selective epoxide ring-opening followed by a lactonization reaction.
Antimalarial activity and toxicity evaluation of a quantified Nauclea pobeguinii extract.[Pubmed:20470876]
J Ethnopharmacol. 2010 Aug 19;131(1):10-6.
AIM OF THE STUDY: To evaluate the in vitro and in vivo antiplasmodial activity and toxicity of the aqueous and 80% EtOH extract of the stem bark of Nauclea pobeguinii (Pob. Ex. Pell.) Petit (Rubiaceae), a plant used in traditional medicine in DR Congo against malaria. MATERIALS AND METHODS: The aqueous and 80% EtOH extract from N. pobeguinii stem bark, and its constituents (5S)-5-Carboxystrictosidine, 19-O-methylangustoline, 3-O-beta-fucosylquinovic acid, 3-ketoquinovic acid and strictosamide, were evaluated for their in vitro activity against Plasmodium falciparum (chloroquine-sensitive Ghana-strain). The 80% EtOH extract, containing 5.6% strictosamide, was evaluated in vivo in the 4-day P. berghei mouse model, and in the P. yoelii N67 model. RESULTS: All compounds were inactive or only moderately active in vitro. The aqueous and 80% EtOH extract displayed moderate in vitro activity with IC(50) values of 44 and 32 microg/mL, respectively, without apparent cytotoxicity on MRC-5 cells (CC50>64 microg/mL). Daily oral dosing of the 80% EtOH extract, at 300 mg/kg, resulted in 86% reduction of parasitaemia in the 4-day P. berghei mouse model, and 75% reduction in the P. yoelii N67 model. Prolonging oral dosing to 2 x 5 days, with an interval of 2 days, and oral administration of the 80% EtOH extract at 300 mg/kg induced 92% reduction of parasitaemia, and a mean survival time of 17 days. Strictosamide, the putative active constituent, may be metabolically activated in the gastrointestinal tract after oral administration. Levels of creatinin, urea, ALAT and ASAT remained unchanged after treatment. No acute toxicity was observed in mice after a single 2g/kg oral dose, nor after 4 weekly doses. No significant macroscopic or microscopic lesions were observed in heart, lung, spleen, kidney, liver, large intestine and brain. CONCLUSIONS: These results can partly support and justify the use of N. pobeguinii in traditional medicine in the DR Congo for the treatment of uncomplicated malaria.
Synthesis and absolute configuration of a new 3,4-dihydro-beta-carboline-type alkaloid, 3,4-dehydro-5(S)-5-carboxystrictosidine, isolated from Peruvian Una de Gato (Uncaria tomentosa).[Pubmed:12372867]
Chem Pharm Bull (Tokyo). 2002 Oct;50(10):1376-8.
The structure including the absolute configuration of a new glucoalkaloid, 3,4-dehydro-5(S)-5-Carboxystrictosidine, isolated from Peruvian Una de Gato (Cat's Claw, original plant: Uncaria tomentosa), was confirmed by synthesis starting from secologanin and L-tryptophan.
A new gluco indole alkaloid, 3, 4-dehydro-5-carboxystrictosidine, from Peruvian Una de Gato (Uncaria tomentosa).[Pubmed:11045440]
Chem Pharm Bull (Tokyo). 2000 Oct;48(10):1410-2.
A new gluco indole alkaloid, 3,4-dehydro-5-Carboxystrictosidine, was obtained from Peruvian Una de Gato (Cat's Claw, original plant: Uncaria tomentosa) together with two known gluco indole alkaloids. This compound was the first example of isolation of a gluco monoterpenoid indole alkaloid having a 3,4-dihydro-beta-carboline ring system from nature. A characteristic feature of the compound was the quick replacement of the methylene hydrogens on C-14 with deuterium that was observed when it was dissolved in CD3OD. We demonstrated a similar proton-deuterium exchange on a model compound, 1-methyl-3,4-dihydro-gamma-carboline, in CD3OD solution.


