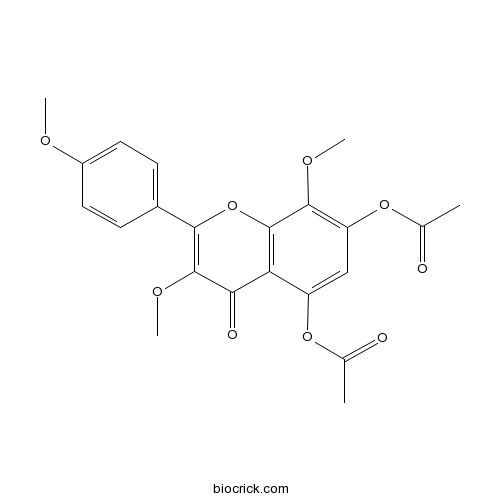
5,7-Diacetoxy-3,4',8-trimethoxyflavoneCAS# 5128-43-8 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 5128-43-8 | SDF | Download SDF |
| PubChem ID | 13916287 | Appearance | Yellow powder |
| Formula | C22H20O9 | M.Wt | 428.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [7-acetyloxy-3,8-dimethoxy-2-(4-methoxyphenyl)-4-oxochromen-5-yl] acetate | ||
| SMILES | CC(=O)OC1=CC(=C(C2=C1C(=O)C(=C(O2)C3=CC=C(C=C3)OC)OC)OC)OC(=O)C | ||
| Standard InChIKey | PKVJLPXCFDHGEY-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5,7-Diacetoxy-3,4',8-trimethoxyflavone Dilution Calculator

5,7-Diacetoxy-3,4',8-trimethoxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3343 mL | 11.6713 mL | 23.3427 mL | 46.6853 mL | 58.3567 mL |
| 5 mM | 0.4669 mL | 2.3343 mL | 4.6685 mL | 9.3371 mL | 11.6713 mL |
| 10 mM | 0.2334 mL | 1.1671 mL | 2.3343 mL | 4.6685 mL | 5.8357 mL |
| 50 mM | 0.0467 mL | 0.2334 mL | 0.4669 mL | 0.9337 mL | 1.1671 mL |
| 100 mM | 0.0233 mL | 0.1167 mL | 0.2334 mL | 0.4669 mL | 0.5836 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Afzelechin 3-O-xyloside
Catalog No.:BCN7774
CAS No.:512781-45-2
- 2,6-Dimethyl-3,7-octadiene-2,6-diol
Catalog No.:BCN5633
CAS No.:51276-34-7
- Gallocatechin gallate
Catalog No.:BCN6803
CAS No.:5127-64-0
- Amsacrine
Catalog No.:BCC4309
CAS No.:51264-14-3
- N-Phthaloyl-Phe-OH
Catalog No.:BCC3016
CAS No.:5123-55-7
- Wighteone
Catalog No.:BCN5632
CAS No.:51225-30-0
- 6,8-Diprenylgenistein
Catalog No.:BCN4805
CAS No.:51225-28-6
- Boc-Arg(Z)-OH
Catalog No.:BCC3068
CAS No.:51219-18-2
- Ascaridole
Catalog No.:BCC8121
CAS No.:512-85-6
- Raffinose
Catalog No.:BCN8427
CAS No.:512-69-6
- Yamogenin
Catalog No.:BCN8277
CAS No.:512-06-1
- Diosgenin
Catalog No.:BCN6272
CAS No.:512-04-9
- 7,4'-Di-O-methylapigenin
Catalog No.:BCN5634
CAS No.:5128-44-9
- L-NAME hydrochloride
Catalog No.:BCC2865
CAS No.:51298-62-5
- Alpha-Eudesmol
Catalog No.:BCC8272
CAS No.:473-16-5
- 27-Hydroxymangiferonic acid
Catalog No.:BCN4626
CAS No.:5132-66-1
- Tizanidine
Catalog No.:BCC4082
CAS No.:51322-75-9
- Aloe-emodin-glucoside
Catalog No.:BCC8130
CAS No.:29010-56-8
- Soyasaponin Bb
Catalog No.:BCN2598
CAS No.:51330-27-9
- Budesonide
Catalog No.:BCC4767
CAS No.:51333-22-3
- delta-Amyrin acetate
Catalog No.:BCN5635
CAS No.:51361-60-5
- Tris DBA
Catalog No.:BCC7685
CAS No.:51364-51-3
- Boc-Methioninol
Catalog No.:BCC2720
CAS No.:51372-93-1
- 2-(2-Aminoethyl)-1-methylpyrrolidine
Catalog No.:BCC8477
CAS No.:51387-90-7
N(6)-Substituted 5'-N-Methylcarbamoyl-4'-selenoadenosines as Potent and Selective A3 Adenosine Receptor Agonists with Unusual Sugar Puckering and Nucleobase Orientation.[Pubmed:28380296]
J Med Chem. 2017 Apr 27;60(8):3422-3437.
Potent and selective A3 adenosine receptor (AR) agonists were identified by the replacement of 4'-oxo- or 4'-thionucleosides with bioisosteric selenium. Unlike previous agonists, 4'-seleno analogues preferred a glycosidic syn conformation and South sugar puckering, as shown in the X-ray crystal structure of 5'-N-methylcarbamoyl derivative 3p. Among the compounds tested, N(6)-3-iodobenzyl analogue 3d was found to be the most potent A3AR full agonist (Ki = 0.57 nM), which was >/=800- and 1900-fold selective for A1AR and A2AAR, respectively. In the N(6)-cycloalkyl series, 2-Cl analogues generally exhibited better hA3AR affinity than 2-H analogues, whereas 2-H > 2-Cl in the N(6)-3-halobenzyl series. N(7) isomers 3t and 3u were much weaker in binding than corresponding N(9) isomers, but compound 3t lacked A3AR activation, appearing to be a weak antagonist. 2-Cl-N(6)-3-iodobenzyl analogue 3p inhibited chemoattractant-induced migration of microglia/monocytes without inducing cell death at
Molecular mechanisms of 3,3'4,4',5-pentachlorobiphenyl-induced epithelial-mesenchymal transition in human hepatocellular carcinoma cells.[Pubmed:28284859]
Toxicol Appl Pharmacol. 2017 May 1;322:75-88.
Polychlorinated biphenyls (PCBs) are classic persistent organic pollutants (POPs). Many studies have found a positive association between the progression of hepatocellular carcinoma (HCC) and PCBs exposure. However, the influence of PCBs on epithelial-mesenchymal transition (EMT) of HCC remains to be unclear. In this study, we explored the effect of PCB126 on EMT in HCC cells and its underlying mechanisms. The data showed that PCB126, exposing both Bel-7402 and SMMC-7721 cells for 48h, promoted EMT that was demonstrated by E-cadherin repression, up-regulation of N-cadherin and vimentin, and morphological alteration. We found that signal transducer and activator of transcription 3 (STAT3)/Snail1 signaling was activated after PCB126 exposure, and the addition of STAT3 inhibitor WP1066 blocked PCB126-induced down-regulation of E-cadherin as well as up-regulation of N-cadherin and vimentin. Moreover, PCB126 exposure increased pyruvate kinase M2 (PKM2) expression and its nuclear translocation, whereas treatment with PKM2 shRNA suppressed the activation of STAT3/Snail1 signaling and the alternation of EMT-related molecules (E-cadherin, N-cadherin and vimentin). Furthermore, this study indicated estrogen receptor (ER) and aryl hydrocarbon receptor (AhR) were involved in PCB126-induced effects on PKM2, STAT3/Snail1 signaling and EMT by according treatment using ER inhibitor ICI and AhR shRNA. Notably, PCB126-increased reactive oxygen species (ROS) production via AhR is associated with activation of PKM2/STAT3/Snail1 cascades and contributes to EMT. Taken together, these results indicated that PCB126 promotes EMT process of HCC cells via PKM2/STAT3/Snail1 signaling which is mediated by ER and AhR.
Design and synthesis of selective CDK8/19 dual inhibitors: Discovery of 4,5-dihydrothieno[3',4':3,4]benzo[1,2-d]isothiazole derivatives.[Pubmed:28302507]
Bioorg Med Chem. 2017 Apr 15;25(8):2336-2350.
To develop a novel series of CDK8/19 dual inhibitors, we employed structure-based drug design using docking models based on a library compound, 4,5-dihydroimidazolo[3',4':3,4]benzo[1,2-d]isothiazole 16 bound to CDK8. We designed various [5,6,5]-fused tricyclic scaffolds bearing a carboxamide group to maintain predicted interactions with the backbone CO and NH of Ala100 in the CDK8 kinase hinge region. We found that 4,5-dihydrothieno[3',4':3,4]benzo[1,2-d]isothiazole derivative 29a showed particularly potent enzymatic inhibitory activity in both CDK8/19 (CDK8 IC50: 0.76nM, CDK19 IC50: 1.7nM). To improve the physicochemical properties and kinase selectivity of this compound, we introduced a substituted 3-pyridyloxy group into the scaffold 8-position. The resulting optimized compound 52h showed excellent in vitro potency (CDK8 IC50: 0.46nM, CDK19 IC50: 0.99nM), physicochemical properties, and kinase selectivity (only 5 kinases showed <35% unbound fraction at 300nM. CDK19: 4.6%, CDK8: 8.3%, HASPIN: 23%, DYRK1B: 27%, HIP1: 32%). Based on a docking model of 52h bound to CDK8, we could explain the highly specific kinase activity profile found for this compound, based on the interaction of the pyridyl group of 52h interacting with Met174 of the CDK8 DMG activation loop. In vitro pharmacological evaluation of 52h revealed potent suppression of phosphorylated STAT1 in various cancer cells. The high oral bioavailability found for this compound enabled in vivo studies, in which we demonstrated a mechanism-based in vivo PD effect as well as tumor growth suppression in an RPMI8226 human hematopoietic and lymphoid xenograft model in mouse [T/C: -1% (2.5mg/kg, qd)].
Discovery of 4-((3'R,4'S,5'R)-6''-Chloro-4'-(3-chloro-2-fluorophenyl)-1'-ethyl-2''-oxodispiro[ cyclohexane-1,2'-pyrrolidine-3',3''-indoline]-5'-carboxamido)bicyclo[2.2.2]octane -1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development.[Pubmed:28339198]
J Med Chem. 2017 Apr 13;60(7):2819-2839.
We previously reported the design of spirooxindoles with two identical substituents at the carbon-2 of the pyrrolidine core as potent MDM2 inhibitors. In this paper we describe an extensive structure-activity relationship study of this class of MDM2 inhibitors, which led to the discovery of 60 (AA-115/APG-115). Compound 60 has a very high affinity to MDM2 (Ki < 1 nM), potent cellular activity, and an excellent oral pharmacokinetic profile. Compound 60 is capable of achieving complete and long-lasting tumor regression in vivo and is currently in phase I clinical trials for cancer treatment.


