3alpha-Hydroxytanshinone IIACAS# 97399-71-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 97399-71-8 | SDF | Download SDF |
| PubChem ID | 14610644 | Appearance | Red powder |
| Formula | C19H18O4 | M.Wt | 310.35 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (7R)-7-hydroxy-1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]benzofuran-10,11-dione | ||
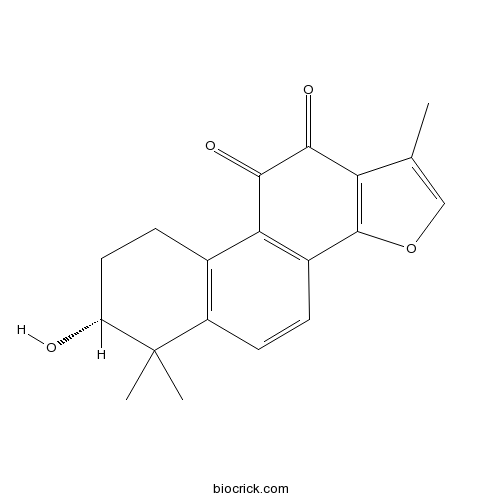
| SMILES | CC1=COC2=C1C(=O)C(=O)C3=C2C=CC4=C3CCC(C4(C)C)O | ||
| Standard InChIKey | PTDUBPDLRWKSBQ-CYBMUJFWSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Phytochemistry, 1985, 24(4):815-817.Pigments from Salvia miltiorrhiza.[Reference: WebLink]
|

3alpha-Hydroxytanshinone IIA Dilution Calculator

3alpha-Hydroxytanshinone IIA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2222 mL | 16.1108 mL | 32.2217 mL | 64.4434 mL | 80.5542 mL |
| 5 mM | 0.6444 mL | 3.2222 mL | 6.4443 mL | 12.8887 mL | 16.1108 mL |
| 10 mM | 0.3222 mL | 1.6111 mL | 3.2222 mL | 6.4443 mL | 8.0554 mL |
| 50 mM | 0.0644 mL | 0.3222 mL | 0.6444 mL | 1.2889 mL | 1.6111 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3222 mL | 0.6444 mL | 0.8055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 8-Hydroxy-4-cadinen-3-one
Catalog No.:BCN4520
CAS No.:97372-53-7
- Troglitazone
Catalog No.:BCC2016
CAS No.:97322-87-7
- Topiramate
Catalog No.:BCC2314
CAS No.:97240-79-4
- Picfeltarraenin IA
Catalog No.:BCN1041
CAS No.:97230-47-2
- Picfeltarraenin IB
Catalog No.:BCN2845
CAS No.:97230-46-1
- Eriobofuran
Catalog No.:BCN7436
CAS No.:97218-06-9
- Meisoindigo
Catalog No.:BCC5132
CAS No.:97207-47-1
- 3-Ethoxyandrosta-3,5-dien-17-one
Catalog No.:BCC8630
CAS No.:972-46-3
- 6-Epi-8-O-acetylharpagide
Catalog No.:BCN4550
CAS No.:97169-44-3
- 6-Geranylnaringenin
Catalog No.:BCN3001
CAS No.:97126-57-3
- AMN 082 dihydrochloride
Catalog No.:BCC7344
CAS No.:97075-46-2
- Porfimer Sodium
Catalog No.:BCC5353
CAS No.:97067-70-4
- Aristolactam AIa
Catalog No.:BCN4854
CAS No.:97399-90-1
- Aristolactam AIIIa
Catalog No.:BCN4521
CAS No.:97399-91-2
- Paniculidine A
Catalog No.:BCN4522
CAS No.:97399-93-4
- Paniculidine B
Catalog No.:BCN4523
CAS No.:97399-94-5
- Paniculidine C
Catalog No.:BCN4524
CAS No.:97399-95-6
- Cynatratoside A
Catalog No.:BCN7087
CAS No.:97399-96-7
- Tanshindiol A
Catalog No.:BCN3123
CAS No.:97411-46-6
- 2',3'-Dehydrosalannol
Catalog No.:BCN4549
CAS No.:97411-50-2
- (-)-Mandelic acid benzyl ester
Catalog No.:BCC8374
CAS No.:97415-09-3
- (-)-5'-DMH-CBD
Catalog No.:BCC5769
CAS No.:97452-63-6
- Cycloart-22-ene-3,25-diol
Catalog No.:BCN4525
CAS No.:97456-49-0
- Tanshindiol B
Catalog No.:BCN3124
CAS No.:97465-70-8
Sodium Tanshinone IIA Sulfonate Prevents Radiation-Induced Toxicity in H9c2 Cardiomyocytes.[Pubmed:28386289]
Evid Based Complement Alternat Med. 2017;2017:4537974.
The present study was designed to elucidate the key parameters associated with X-ray radiation induced oxidative stress and the effects of STS on X-ray-induced toxicity in H9c2 cardiomyocytes. Cytotoxicity of STS and radiation was assessed by MTT. Antioxidant activity was evaluated by SOD and MDA. Apoptosis was measured by the flow cytometry, Hoechst 33258, clonogenic survival assay, and western blot. It was found that the cell viability of H9c2 cells exposed to X-ray radiation was significantly decreased in a dose-dependent manner and was associated with cell cycle arrest at the G0/G1 phase as well as apoptosis. STS treatment significantly reversed the morphological changes, attenuated radiation-induced apoptosis, and improved the antioxidant activity in the H9c2 cells. STS significantly increased the Bcl-2 and Bcl-2/Bax levels and decreased the Bax and caspase-3 levels, compared with the cells treated with radiation alone. STS treatment also resulted in a significant increase in p38-MAPK activation. STS could protect the cells from X-ray-induced cell cycle arrest, oxidative stress, and apoptosis. Therefore, we suggest the STS could be useful for the treatment of radiation-induced cardiovascular injury.
Fludarabine resistance mediated by aminoglycoside-3'-phosphotransferase-IIa and the structurally related eukaryotic cAMP-dependent protein kinase.[Pubmed:28373209]
FASEB J. 2017 Jul;31(7):3007-3017.
While working with G418-resistant stably transfected cells, we realized the neomycin resistance (NeoR) gene, which encodes the aminoglycoside-3'-phosphotransferase-IIa [APH(3')-IIa], also confers resistance to the nucleoside analog fludarabine. Fludarabine is a cytostatic drug widely used in the treatment of hematologic and solid tumors, as well as in the conditioning of patients before transplantation of hematopoietic progenitors. We present evidence that NeoR-transfected cells do not incorporate fludarabine, thus avoiding DNA damage caused by the drug, evidenced by a lack of FANCD2 monoubiquitination and impaired apoptosis. A screening of other nucleoside analogs revealed that APH(3')-IIa only protects against ATP purine analogs. Moreover, APH(3')-IIa ATPase activity is inhibited by fludarabine monophosphate, suggesting that APH(3')-IIa blocks fludarabine incorporation into DNA by dephosphorylating its active fludarabine triphosphate form. Furthermore, overexpression of the catalytic subunit of the eukaryotic kinase PKA, which is structurally related to APHs, also provides resistance to fludarabine, anticipating its putative utility as a response marker to the drug. Our results preclude the use of Neo marker plasmids in the study of purine analogs and unveils a new resistance mechanism against these chemotherapeuticals.-Sanchez-Carrera, D., Bravo-Navas, S., Cabezon, E., Arechaga, I., Cabezas, M., Yanez, L., Pipaon, C. Fludarabine resistance mediated by aminoglycoside-3'-phosphotransferase-IIa and the structurally related eukaryotic cAMP-dependent protein kinase.
The MOBILE Study-A Phase IIa Enriched Enrollment Randomized Withdrawal Trial to Assess the Analgesic Efficacy and Safety of ASP8477, a Fatty Acid Amide Hydrolase Inhibitor, in Patients with Peripheral Neuropathic Pain.[Pubmed:28383710]
Pain Med. 2017 Dec 1;18(12):2388-2400.
Objective: To evaluate the analgesic efficacy and safety of ASP8477 in patients with peripheral neuropathic pain (PNP). Design: Enriched enrollment randomized withdrawal. Setting: Centers in Poland (four), Czech Republic (six), and the United Kingdom (two). Subjects: Patients aged 18 years or older with PNP resulting from painful diabetic peripheral neuropathy or postherpetic neuralgia. Methods: A four-week screening period followed by a single-blind period (six-day dose titration and three-week maintenance period with ASP8477 [20/30 mg BID]). Treatment responders (defined as a >/=30% decrease in the mean average daily pain intensity during the last three days of the single-blind period) were stratified by disease and randomized to receive placebo or continue ASP8477 during a three-week, double-blind, randomized withdrawal period. The primary end point was change in mean 24-hour average numeric pain rating scale (NPRS) from baseline to end of double-blind period. Results: Among 132 patients who enrolled, 116 entered the single-blind period and 63 (ASP8477, N = 31; placebo, N = 32) completed the double-blind period. There was no difference in mean 24-hour average NPRS score (P = 0.644) or in time-to-treatment failure (P = 0.485) between ASP8477 and placebo. During the single-blind period, 57.8% of patients were treatment responders. ASP8477 was well tolerated. During the single-blind period, 22% of patients experienced at least one treatment-related adverse event (TEAE); during the double-blind period, 8% in the ASP8477 arm and 18% in the placebo arm experienced at least one TEAE. Conclusions: ASP8477 was well tolerated in patients with PNP; however, ASP8477 did not demonstrate a significant treatment difference compared with placebo.
Randomized study between radical surgery and radiotherapy for the treatment of stage IB-IIA cervical cancer: 20-year update.[Pubmed:28382797]
J Gynecol Oncol. 2017 May;28(3):e34.
OBJECTIVE: Stage IB-IIA cervical carcinoma can be equally cured either by radical surgery or radiotherapy (RT). Albeit such policies show the same efficacy, they carry a different morbidity. This is an update after 20 years of a previously published randomized trial of RT vs. surgery in the treatment of stage IB-IIA cervical cancers to assess long-term survival and morbidity and the different pattern of relapse between the 2 modalities. METHODS: Between September 1986 and December 1991, women referred for a newly diagnosed stage IB and IIA cervical carcinoma were randomized to radical surgery or RT. The primary outcome measures were long-term survival and complications rate. The secondary outcome was recurrence of the disease. RESULTS: Three-hundred forty-three eligible women were randomized: 172 to radical surgery and 171 to external RT. Minimum follow-up was 19 years. Thirty-three patients (10%) died of intercurrent disease (31 cases) or fatal complications (2 cases). Twenty-year overall survival is 72% and 77% in the 2 treatment groups (p=0.280), respectively. As a whole, 94 recurrences (28%) were observed. Median time to relapse was 13.5 (surgery group) and 11.5 months (radiotherapy group) (p=0.100), respectively. Multivariate analysis confirms that risk factors for survival are histotype (p=0.020), tumor diameter (p=0.008), and lymph node status (p<0.001). CONCLUSION: The results of the present study seem to suggest that there is no treatment of choice for early stage cervical carcinoma in terms of survival. Long term follow-up confirms that the best treatment for the individual patient should take into account clinical factors such as menopausal status, comorbidities, histological type, and tumor diameter.


