3Beta-Isodihydrocadambine 4-oxideCAS# 1092371-18-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1092371-18-0 | SDF | Download SDF |
| PubChem ID | 102004692 | Appearance | Powder |
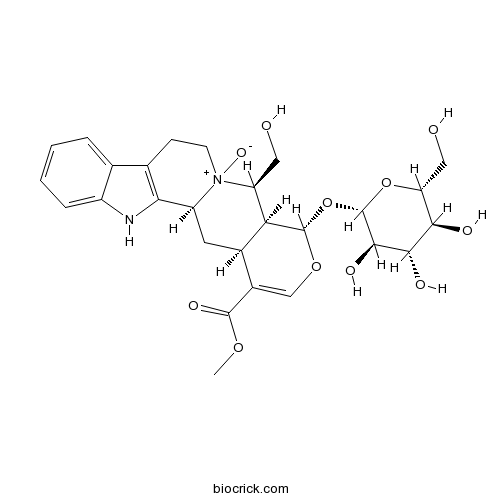
| Formula | C27H34N2O11 | M.Wt | 562.6 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | COC(=O)C1=COC(C2C1CC3C4=C(CC[N+]3(C2CO)[O-])C5=CC=CC=C5N4)OC6C(C(C(C(O6)CO)O)O)O | ||
| Standard InChIKey | NVUHVENKCFNJQW-GDPYHQKMSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Structure Identification | Helvetica Chimica Acta, 2008, 91(11):2148-2152.Indole Alkaloids from the Leaves of Anthocephalus chinensis.[Reference: WebLink]
|

3Beta-Isodihydrocadambine 4-oxide Dilution Calculator

3Beta-Isodihydrocadambine 4-oxide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7775 mL | 8.8873 mL | 17.7746 mL | 35.5492 mL | 44.4365 mL |
| 5 mM | 0.3555 mL | 1.7775 mL | 3.5549 mL | 7.1098 mL | 8.8873 mL |
| 10 mM | 0.1777 mL | 0.8887 mL | 1.7775 mL | 3.5549 mL | 4.4437 mL |
| 50 mM | 0.0355 mL | 0.1777 mL | 0.3555 mL | 0.711 mL | 0.8887 mL |
| 100 mM | 0.0178 mL | 0.0889 mL | 0.1777 mL | 0.3555 mL | 0.4444 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Poziotinib
Catalog No.:BCC6380
CAS No.:1092364-38-9
- PP242
Catalog No.:BCC3682
CAS No.:1092351-67-1
- PSB 603
Catalog No.:BCC7598
CAS No.:1092351-10-4
- Camellianin A
Catalog No.:BCN7864
CAS No.:109232-77-1
- ent-11beta-Hydroxyatis-16-ene-3,14-dione
Catalog No.:BCN6600
CAS No.:1092103-22-4
- 3,4,5-Trimethoxyphenyl-(6-O-galloyl)-O-beta-D-glucopyranoside
Catalog No.:BCN7272
CAS No.:109206-94-2
- Tachioside
Catalog No.:BCN5884
CAS No.:109194-60-7
- Boc-Chg-OH
Catalog No.:BCC3163
CAS No.:109183-71-3
- 2-(Chloromethyl)-4-methylquinazoline
Catalog No.:BCC8482
CAS No.:109113-72-6
- Icariside B1
Catalog No.:BCN7271
CAS No.:109062-00-2
- Schizanthine E
Catalog No.:BCN1937
CAS No.:109031-04-1
- CGS 12066B dimaleate
Catalog No.:BCC6732
CAS No.:109028-10-6
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- NVP-BSK805
Catalog No.:BCC1815
CAS No.:1092499-93-8
- Deuterated Atazanivir-D3-2
Catalog No.:BCC2116
CAS No.:1092540-51-6
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Paxiphylline D
Catalog No.:BCN5885
CAS No.:1092555-02-6
- Paxiphylline E
Catalog No.:BCN5886
CAS No.:1092555-03-7
- (3R,4S)-Tofacitinib
Catalog No.:BCC4268
CAS No.:1092578-46-5
- (3S,4S)-Tofacitinib
Catalog No.:BCC4052
CAS No.:1092578-47-6
- (3S,4R)-Tofacitinib
Catalog No.:BCC4267
CAS No.:1092578-48-7
- WAY 100635 hydrochloride
Catalog No.:BCC5061
CAS No.:146714-97-8
- IT1t dihydrochloride
Catalog No.:BCC6234
CAS No.:1092776-63-0
2,2'-Sulfonyl-dipyrazine 4-oxide.[Pubmed:22798929]
Acta Crystallogr Sect E Struct Rep Online. 2012 Jul 1;68(Pt 7):o2285.
In the title compound, C(8)H(6)N(4)O(3)S, the dihedral angle between the pyrazine rings is 85.04 (1) degrees . In the crystal, mol-ecules are arranged along the a axis and are linked by C-Hcdots, three dots, centeredN hydrogen bonds and pyrazine-pyrazine pi-pi inter-actions [centroid-centroid distance = 3.800 (1) A, forming an infinite chain array. The chains are connected by C-Hcdots, three dots, centeredO(oxide) hydrogen bonds into layers lying parallel to the ab plane. Along the c axis, the layers are stacked and linked through C-Hcdots, three dots, centeredO(sulfon-yl) inter-actions, forming a three-dimensional network.
3-Methyl-quinoxaline-2-carb-oxy-lic acid 4-oxide monohydrate.[Pubmed:21588010]
Acta Crystallogr Sect E Struct Rep Online. 2010 Jun 26;66(Pt 7):o1801.
In the crystal structure of the title compound, C(10)H(8)N(2)O(3).H(2)O, mol-ecules are linked via inter-molecular O-Hcdots, three dots, centeredO and O-Hcdots, three dots, centeredN hydrogen bonds into a two-dimensional network.
La0.3Sr0.2Mn0.1Zn0.4 oxide-Sm0.2Ce0.8O1.9 (LSMZ-SDC) nanocomposite cathode for low temperature SOFCs.[Pubmed:22905565]
J Nanosci Nanotechnol. 2012 Jun;12(6):4994-7.
Nanocomposite based cathode materials compatible for low temperature solid oxide fuel cells (LTSOFCs) are being developed. In pursuit of compatible cathode, this research aims to synthesis and investigation nanocomposite La0.3Sr0.2Mn0.1Zn0.4 oxide-Sm0.2Ce0.8O1.9 (LSMZ-SDC) based system. The material was synthesized through wet chemical method and investigated for oxide-ceria composite based electrolyte LTSOFCs. Electrical property was studied by AC electrochemical impedance spectroscopy (EIS). The microstructure, thermal properties, and elemental analysis of the samples were characterized by TGA/DSC, XRD, SEM, respectively. The AC conductivity of cathode was obtained for 2.4 Scm(-1) at 550 degrees C in air. This cathode is compatible with ceria-based composite electrolytes and has improved the stability of the material in SOFC cathode environment.
Vibrational spectroscopy investigation using ab initio and DFT vibrational analysis of 7-chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepine-4-oxide.[Pubmed:23732619]
Spectrochim Acta A Mol Biomol Spectrosc. 2013 Sep;113:224-35.
The FT-IR and FT-Raman spectrum of 7-chloro-2-methylamino-5-phenyl-3H-1, 4-benzodiazepine-4-oxide (7CMP4BO) has been recorded in the region 4000-400 and 4000-100 cm(-1) respectively. The optimized geometry, Thermodynamic properties, NBO, Molecular Electrostatic Potentials, PES, frequency and intensity of the vibrational bands of 7CMP4BO were obtained by the ab initio HF and density functional theory (DFT), B3LYP/6-31G (d,p) basis set. The molecule orbital contributions were studied by using the total (TDOS), partial (PDOS), and overlap population (OPDOS) density of states. The harmonic vibrational frequencies were calculated and the scaled values have been compared with experimental FT-IR and FT-Raman spectra. A detailed interpretation of the vibrational spectra of this compound has been made on the basis of the calculated potential energy distribution (PED). The linear polarizability (alpha) and the first order hyperpolarizability (beta) values of the investigated molecule have been computed using DFT quantum mechanical calculations. The observed and the calculated frequencies are found to be in good agreement. The experimental spectra also coincide satisfactorily with those of theoretically calculated values.


